911798
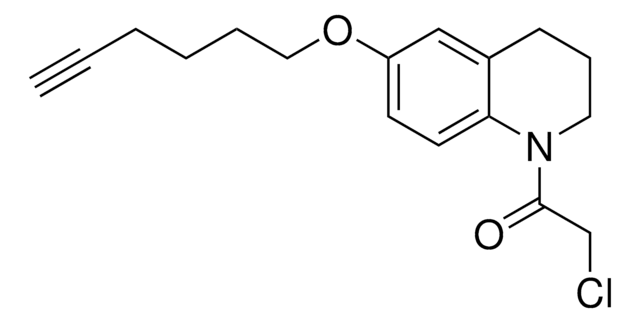
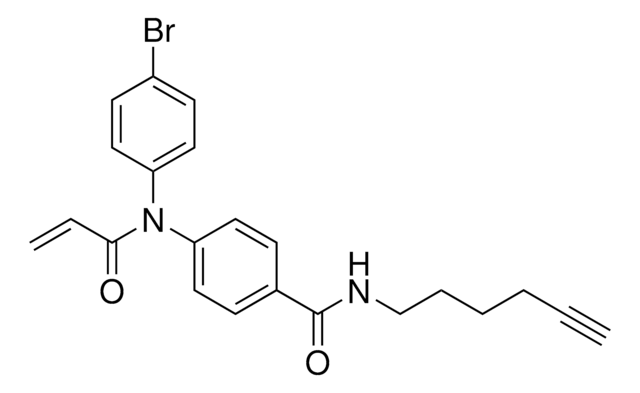
N-(4-Bromophenyl)-N-phenylacrylamide
≥95%
Synonym(s):
Electrophilic scout fragment, KB05, Scout fragment for targetable cysteine
About This Item
Recommended Products
Application
Other Notes
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Ligandability describes the propensity of a protein target to bind a small molecule with high affinity. It is a precursor to evaluating druggability, which requires more advanced translational pharmacological effects and drug-like properties in vivo.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service