84737
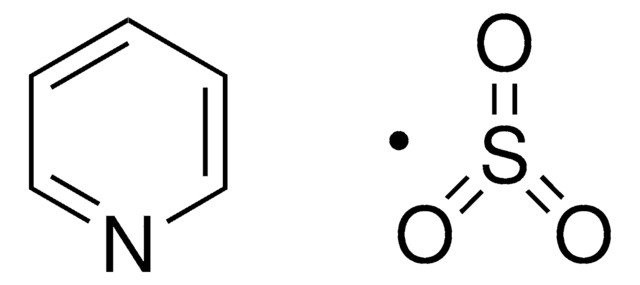
Sulfur trioxide pyridine complex
technical, ≥45% SO3 basis
Synonym(s):
NSC 75831, Pyridine sulfur trioxide complex
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H5NO3S
CAS Number:
Molecular Weight:
159.16
Beilstein:
3704116
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
grade
technical
form
powder
reaction suitability
reagent type: oxidant
concentration
≥45% (SO3)
ign. residue
≤0.05%
mp
154-170 °C
SMILES string
O=S(=O)=O.c1ccncc1
InChI
1S/C5H5N.O3S/c1-2-4-6-5-3-1;1-4(2)3/h1-5H;
InChI key
UDYFLDICVHJSOY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Sulfation agent
Reactant for:
- Preparation of azido anologs of pregnanolone
- Sulfate esters of morphine derivatives
- Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents
Sulfur trioxide pyridine complex is mainly a sulfation agent that can be used for sulfations of alcohols, sulfonations, deoxygenations and other reductions, oxidations with DMSO.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies on Selectin Blocker. 1. Structure- Activity Relationships of Sialyl Lewis X Analogs.
Ohmoto H, et al.
Journal of Medicinal Chemistry, 39(6), 1339-1343 (1996)
G.A. Olah et al.
Synthesis, 59-59 (1979)
In vitro and in vivo selectin-blocking activities of sulfated lipids and sulfated sialyl compounds.
Mulligan MS, et al.
International Immunology, 10(5), 569-575 (1998)
Synthesis, 984-984 (1979)
Sulfated hyaluronan derivatives reduce the proliferation rate of primary rat calvarial osteoblasts.
Kunze R, et al.
Glycoconjugate Journal, 27(1), 151-158 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service