810002
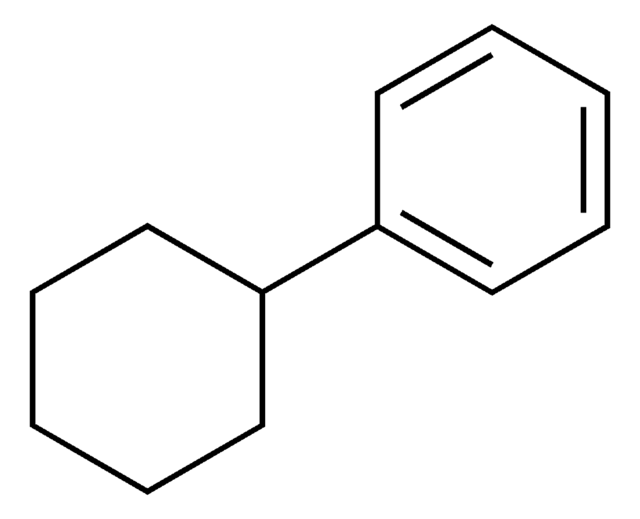
Phenylcyclohexane
battery grade, ≥99%, acid < 200 ppm, H2O < 100 ppm
Synonym(s):
Cyclohexylbenzene
About This Item
Recommended Products
grade
battery grade
Quality Level
Assay
≥99%
form
liquid
impurities
≤100 ppm H2O
≤200 ppm acid
refractive index
n20/D 1.526 (lit.)
bp
239-240 °C (lit.)
mp
4-7 °C (lit.)
density
0.95 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
C1CCC(CC1)c2ccccc2
InChI
1S/C12H16/c1-3-7-11(8-4-1)12-9-5-2-6-10-12/h1,3-4,7-8,12H,2,5-6,9-10H2
InChI key
IGARGHRYKHJQSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Legal Information
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Here, we present a short review of ionic liquid electrolytes used in state-of-the-art rechargeable batteries including high performance and low-cost aluminum batteries, non-flammable Li-based batteries, and high-cycling and stable dual-graphite batteries. We also outline the key issues explored so as to identify the future direction of IL development.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service