77861
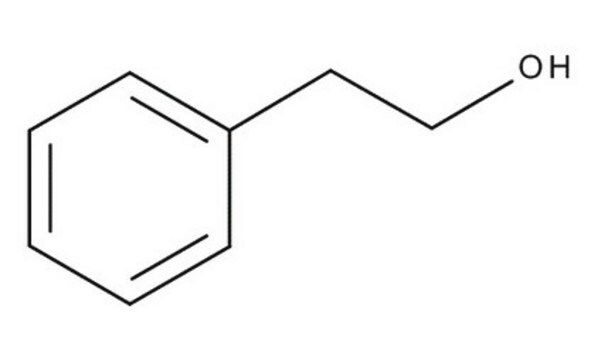
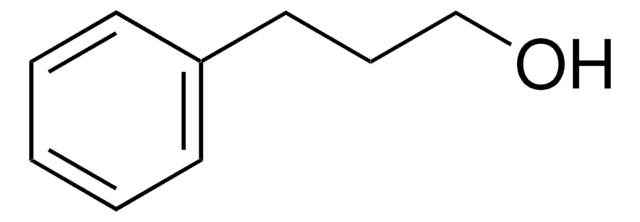
2-Phenylethanol
≥99.0% (GC)
Synonym(s):
β-PEA, 2-Phenylethyl alcohol, Benzyl carbinol, PEA
About This Item
Recommended Products
vapor density
4.21 (vs air)
vapor pressure
1 mmHg ( 58 °C)
Assay
≥99.0% (GC)
form
liquid
refractive index
n20/D 1.5317 (lit.)
bp
219-221 °C/750 mmHg (lit.)
mp
−27 °C (lit.)
density
1.020 g/mL at 20 °C (lit.)
functional group
hydroxyl
phenyl
SMILES string
OCCc1ccccc1
InChI
1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2
InChI key
WRMNZCZEMHIOCP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- The bacteriostatic activity of 2-phenylethanol derivatives correlates with membrane binding affinity: This study examines how the bacteriostatic properties of 2-phenylethanol derivatives are influenced by their affinity for binding to membranes, providing insights into their antimicrobial effectiveness (IS Kleinwächter et al., 2021).
- Anti-depressive-like effect of 2-phenylethanol inhalation in mice: This study explores the neuropsychological effects of inhaling 2-phenylethanol, suggesting potential therapeutic applications for depression (H Ueno et al., 2019).
recommended
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service