733539
N-Boc-pyrrole-2-boronic acid MIDA ester
95%
Synonym(s):
1-(tert-Butoxycarbonyl)pyrrole-2-boronic acid MIDA ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H19BN2O6
CAS Number:
Molecular Weight:
322.12
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
166-171 °C
SMILES string
CN1CC(=O)OB(OC(=O)C1)c2cccn2C(=O)OC(C)(C)C
InChI
1S/C14H19BN2O6/c1-14(2,3)21-13(20)17-7-5-6-10(17)15-22-11(18)8-16(4)9-12(19)23-15/h5-7H,8-9H2,1-4H3
InChI key
HASMDYSWFBSATG-UHFFFAOYSA-N
Application
N-Boc-pyrrole-2-boronic acid MIDA ester can be used:
- As a starting material for the synthesis of marine natural product pentabromopseudilin.
- To prepare 5,5′-(3,4-dihexylthiophene-2,5-diyl)bis(1H-pyrrole-2-carbaldehyde), a key intermediate for the synthesis of thiophene based novel macrocycles.
- As a substrate in the palladium-catalyzed one-pot meta arylation of bromo substituted 2-phenylpyridine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Two-step total synthesis of an anti-MRSA and myosin-inhibiting marine natural product pentabromopseudilin via Suzuki-Miyaura coupling of a MIDA boronate ester
Kum D, et al.
Tetrahedron Letters, 58(34), 3374-3376 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service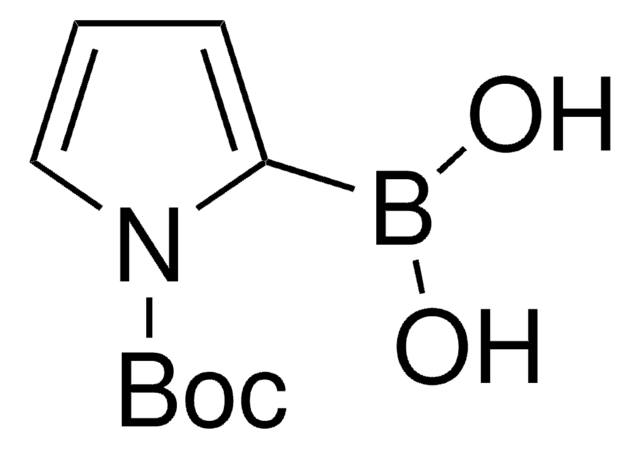

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
