653322
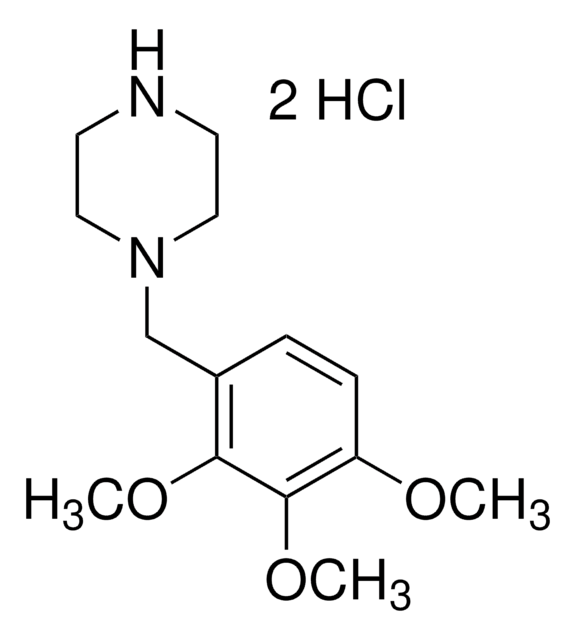
1-(2,3,4-Trimethoxybenzyl)piperazine dihydrochloride
97%
Synonym(s):
Trimetazidine dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H22N2O3 · 2HCl
CAS Number:
Molecular Weight:
339.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
231-235 °C (lit.)
SMILES string
Cl[H].Cl[H].COc1ccc(CN2CCNCC2)c(OC)c1OC
InChI
1S/C14H22N2O3.2ClH/c1-17-12-5-4-11(13(18-2)14(12)19-3)10-16-8-6-15-7-9-16;;/h4-5,15H,6-10H2,1-3H3;2*1H
InChI key
VYFLPFGUVGMBEP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-(2,3,4-Trimethoxybenzyl)piperazine dihydrochloride or trimetazidine dihydrochloride can be used as a building block to synthesize:
- Phenylpropyl trimetazidine derivatives with potent cerebral vasodilator activity.
- Benzoylguanidine-trimetazidine derivatives for myocardial ischemic-reperfusion activity studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Benzylpiperazine Derivatives. I. Syntheses and Biological Activities of 1 (2, 3, 4-Trimethoxybenzyl) piperazine Derivatives
OHTAKA H, et al.
Chemical & Pharmaceutical Bulletin, 35(7), 2774-2781 (1987)
Design, synthesis and biological evaluation of novel substituted benzoylguanidine derivatives as potent Na+/H+ exchanger inhibitors
Xu W-T, et al.
Bioorganic & Medicinal Chemistry Letters, 19(12), 3283-3287 (2009)
Le Tran Phuc Khoa et al.
Cell stem cell, 27(3), 441-458 (2020-07-02)
Self-renewing embryonic stem cells (ESCs) respond to environmental cues by exiting pluripotency or entering a quiescent state. The molecular basis underlying this fate choice remains unclear. Here, we show that histone acetyltransferase MOF plays a critical role in this process
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 653322-25G | 4061832732794 |
| 653322-10G | 4061832732787 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service