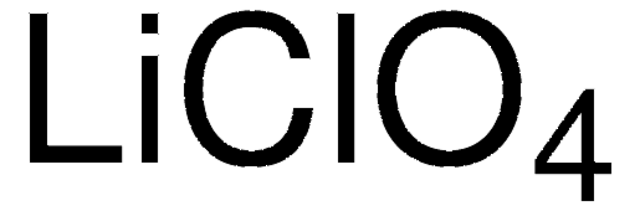634565
Lithium perchlorate
battery grade, dry, 99.99% trace metals basis
Synonym(s):
Lithium cloricum, Perchloric acid, lithium salt
About This Item
Recommended Products
grade
for analytical purposes
Assay
99.99% trace metals basis
form
powder and chunks
reaction suitability
reagent type: oxidant
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
236 °C (lit.)
solubility
H2O: 106.4 g/L at 20 °C
application(s)
battery manufacturing
greener alternative category
SMILES string
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI key
MHCFAGZWMAWTNR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
accessory
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nanomaterials for Energy Storage in Lithium-ion Battery Applications
Increasing fuel costs and concerns about greenhouse gas emissions have spurred the growth in sales of hybrid electric vehicles (HEVs) that carry a battery pack to supplement the performance of the internal combustion engine (ICE).
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Discover more about advancements being made to improve energy density of lithium ion battery materials.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service