591130
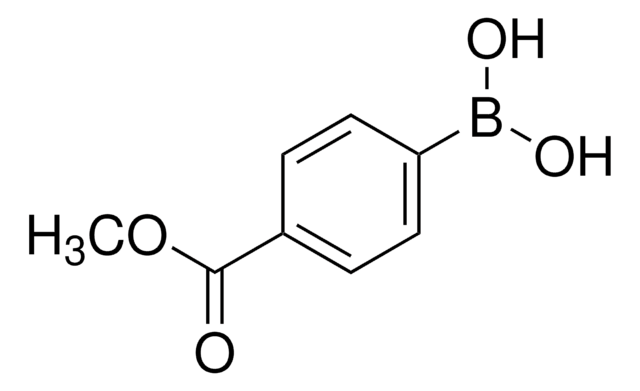
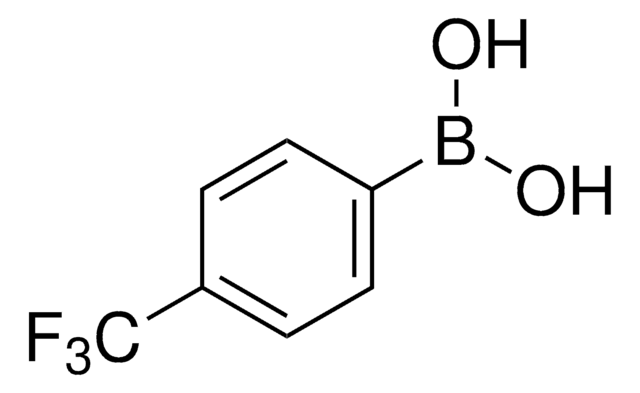
3-Methoxycarbonylphenylboronic acid
Synonym(s):
Methyl 3-boronobenzoate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3O2CC6H4B(OH)2
CAS Number:
Molecular Weight:
179.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
205-208 °C (lit.)
functional group
ester
SMILES string
COC(=O)c1cccc(c1)B(O)O
InChI
1S/C8H9BO4/c1-13-8(10)6-3-2-4-7(5-6)9(11)12/h2-5,11-12H,1H3
InChI key
ALTLCJHSJMGSLT-UHFFFAOYSA-N
Application
Reactant involved in:
- Suzuki-Miyaura cross-coupling
- Iterative cross-coupling of boronate building blocks
- Cross-coupling with aryl / alkenyl sulfonates
- Synthesis of symmetrical biaryls via CuCl catalyzed homocoupling
- Trifluoromethylation
- Cyanation
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhichun Shi et al.
Polymers, 11(11) (2019-11-27)
Four kinds of newly synthesized achiral phenylacetylenes bearing a phenylhydrogalvinoxyl residue at 4-position were polymerized by using a chiral rhodium catalyst system, [Rh(nbd)B(C6H5)4] or [Rh(nbd)Cl]2 catalysts in the presence of chiral (R)-(+)- or (S)-(-)-1-phenylethylamine ((R)- or (S)-PEA) cocatalysts. Poly(m-HGDHPA) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service