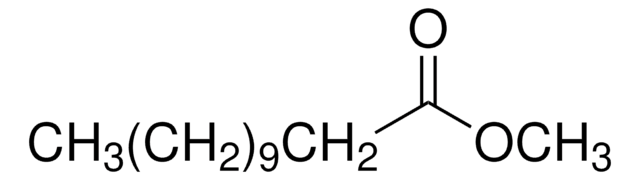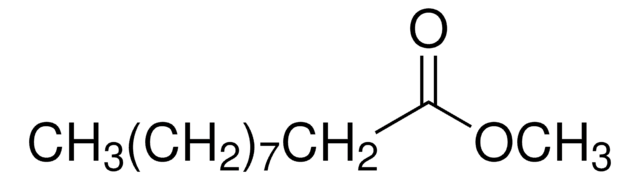49661
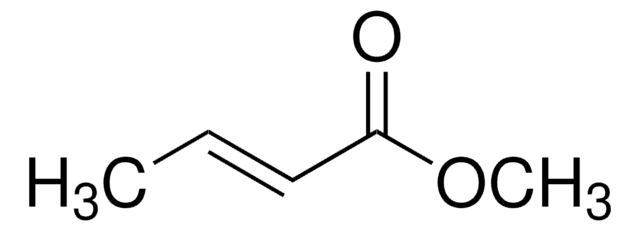
Methyl 4-pentenoate
≥95.0% (GC)
Synonym(s):
4-Pentenoic acid methyl ester, Allylacetic acid methyl ester, Methyl allylacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10O2
CAS Number:
Molecular Weight:
114.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (GC)
refractive index
n20/D 1.415
n20/D 1.415
functional group
allyl
ester
SMILES string
COC(=O)CCC=C
InChI
1S/C6H10O2/c1-3-4-5-6(7)8-2/h3H,1,4-5H2,2H3
InChI key
SHCSFZHSNSGTOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl 4-pentenoate is an unsaturated methyl ester that is prepared by the esterification of 1-pentenoic acid with methanol. It undergoes amidation with formamide via acetone-initiated photochemical reaction to form a 1:1 adduct. The metathesis of methyl 4-pentenoate with different Mo(VI)alkylidene complexes have been reported. It acts as a chain transfer agent during the polymerization of exo,exo-5,6-bis(methoxymethyl)-7-oxabicyclo[2.2.1]hept-2-ene using RuII(H20)6(tos)2 (tos =p-toluenesulfonate) as a catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.2 °F - closed cup
Flash Point(C)
29 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Light-Induced Amidation of Terminal Olefins1.
Elad D and Rokach J.
The Journal of Organic Chemistry, 29(7), 1855-1859 (1964)
Chain transfer during the aqueous ring-opening metathesis polymerization of 7-oxanorbornene derivatives.
France MB, et al.
Macromolecules, 26(18), 4742-4747 (1993)
Coupling of terminal olefins by molybdenum (VI) imido alkylidene complexes.
Fox HH, et al.
Organometallics, 13(2), 635-639 (1994)
Majd Al-Naji et al.
ChemSusChem, 12(12), 2628-2636 (2019-04-18)
The need for more sustainable products and processes has led to the use of new methodologies with low carbon footprints. In this work, an efficient tandem process is demonstrated for the liquid-phase catalytic upgrading of lignocellulosic biomass-derived γ-valerolactone (GVL) with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service