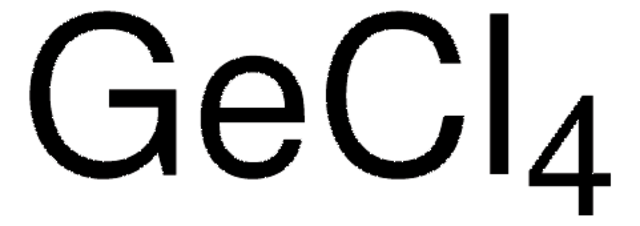483702
Germanium(IV) oxide
≥99.99% trace metals basis
Synonym(s):
Germanium dioxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
GeO2
CAS Number:
Molecular Weight:
104.64
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
powder
mp
>400 °C (lit.)
solubility
ethylene glycol: soluble
density
4.23 g/cm3
application(s)
battery manufacturing
SMILES string
O=[Ge]=O
InChI
1S/GeO2/c2-1-3
InChI key
YBMRDBCBODYGJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Germanium(VI) oxide, also known as germania or germanium dioxide, is a white or off-white powder. It is a refractory metal oxide with a high melting point (1,600 °C) and offers excellent chemical resistance. In addition, germanium(VI) oxide has excellent optoelectronic properties that have made it a valuable material in the semiconducting industry. It has a high refractive index of approximately 2.1, which is higher than that of many other semiconductor materials, and a low absorption coefficient. These properties make it useful for applications such as waveguides, antireflective coatings, and high-index contrast coatings. In addition, germanium(VI) has a high bandgap of about 3.1 eV coupled with relatively high transparency in the infrared region of the electromagnetic spectrum, which makes it useful for applications such as infrared detectors, LEDs, and solar cells.
Application
Building block for inorganic nanowires. Used in the synthesis of ultra-high refractive index and ultra-low loss optical waveguides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rao, C.N.R., et al.
Journal of Materials Chemistry, 14, 440-440 (2004)
Imoto, K.
Optronics, 260, 116-116 (2003)
Jie Liang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(71), 17879-17884 (2017-11-10)
The attractive properties of layered inorganic materials, which make them suitable for numerous applications in chemical industries and life sciences, originated from their crystalline framework structures. Here, we report a new layered germanate PKU-21, which was prepared by the hydrothermal
Xinguo Hong et al.
The Review of scientific instruments, 80(7), 073908-073908 (2009-08-07)
We describe an approach for acquiring high quality x-ray absorption fine structure (XAFS) spectroscopy spectra with wide energy range at high pressure using diamond anvil cell (DAC). Overcoming the serious interference of diamond Bragg peaks is essential for combining XAFS
Sarah L Sewell et al.
Dalton transactions (Cambridge, England : 2003), (29)(29), 3857-3865 (2008-07-17)
Biomimetic synthesis is emerging as an advantageous alternative to the harsh synthetic conditions traditionally used in metal oxide syntheses techniques. Silaffins, proteins from the C. fusiformis diatom, form silica in an aqueous environment under benign conditions. Amine terminated PAMAM and
Articles
Advanced Inorganic Materials for Solid State Lighting
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 483702-25G | 4061832390109 |
| 483702-5G | 4061832390154 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service