All Photos(1)
About This Item
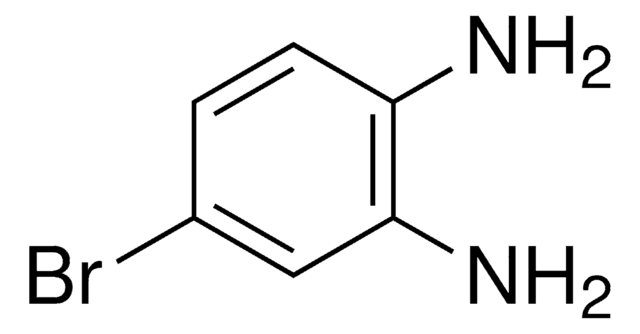
Linear Formula:
C6H5N[CH(CH3)2]2
CAS Number:
Molecular Weight:
177.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.519 (lit.)
bp
95-96 °C/11 mmHg (lit.)
density
0.91 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CC(C)N(C(C)C)c1ccccc1
InChI
1S/C12H19N/c1-10(2)13(11(3)4)12-8-6-5-7-9-12/h5-11H,1-4H3
InChI key
OVSARSKQWCLSJT-UHFFFAOYSA-N
General description
N,N-Diisopropylaniline is an N,N-dialkylaniline. Its borane adducts have been prepared and reported as hydroborating agents. Thermal dissociation of the borane-N,N-diisopropylaniline adduct has been reported to afford gaseous diborane. N,N-Diisopropylaniline is a diisoproplyamino derivative that can be prepared by reacting bromobenzene with diisopropylamine.
Application
N,N-Diisopropylaniline was used for the synthesis of 4-diisopropylamino benzonitrile. It may be used for the synthesis of 6-(4-bromophenyl)-3-methoxy-5-methyl-8-oxabicyclo[3.2.1]octa-3,6-dien-2-one and N,N,N′,N′-tetraisopropylbenzidine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yvonne D Williams et al.
The Journal of organic chemistry, 78(23), 11707-11713 (2013-11-01)
Methoxytropolones are useful scaffolds for therapeutic development because of their known biological activity and established value in the synthesis of α-hydroxytropolones. Upon treatment with triflic acid, a series of 3-methoxy-8-oxabicyclo[3.2.1]octa-3,6-dien-2-ones rearrange rapidly and cleanly to form methoxytropolones. Interestingly, bicycles that
N-alkylation of hindered secondary aromatic amines with 2-iodobutane.
Katritzky AR, et al.
ORL; Journal for Oto-Rhino-Laryngology and Its Related Specialties, 23(4), 399-402 (1991)
Synthesis of N,N,N',N'-tetraalkylbenzidines through oxidative coupling of N,N-dialkylarylamines induced by SbCl5.
Vitale P, et al.
ARKIVOC (Gainesville, FL, United States), 3, 36-48 (2013)
Dual fluorescence and fast intramolecular charge transfer with 4-(diisopropylamino) benzonitrile in alkane solvents.
Demeter A, et al.
Chemical Physics Letters, 323(3), 351-360 (2000)
Brown et al.
The Journal of organic chemistry, 65(15), 4655-4661 (2000-08-26)
Several N,N-diethyl-tert-alkylamines, such as N,N-diethyl-2-methyl-2-butylamine (1, t-PentNEt2), N,N-diethyl-2,3-dimethyl-2-butylamine (2, t-HexNEt2), N,N-diethyl-2,3,3-trimethyl-2-butylamine (3, t-HeptNEt2), and N,N-diethyl-1,1,3,3-tetramethylbutylamine (4, t-OctNEt2) with varying steric bulk around nitrogen (by changing the tert-alkyl group) have been prepared and examined as borane carriers. The complexing ability of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service