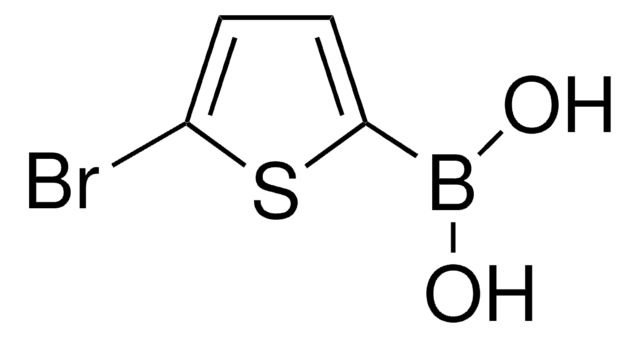
436836
2-Thienylboronic acid
≥95.0%
Synonym(s):
2-Thienylboric acid, 2-Thienylboronic acid, Thien-5-ylboronic acid, Thiophene-2-boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H5BO2S
CAS Number:
Molecular Weight:
127.96
Beilstein:
112375
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
138-140 °C (lit.)
storage temp.
2-8°C
SMILES string
OB(O)c1cccs1
InChI
1S/C4H5BO2S/c6-5(7)4-2-1-3-8-4/h1-3,6-7H
InChI key
ARYHTUPFQTUBBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used for
Reagent used in Preparation of
- Palladium-catalyzed Suzuki-Miyaura cross-couplings
- Alkylation, boration, coupling reaction, Suzuki coupling, and halogenation of fluorenyl bromide
- Chain-growth catalyst transfer polycondensation of conjugated alternating copolymer
- Ferric perchlorate-promoted reaction of fullerene to give fullerenyl boronic esters
- Ligand-free Suzuki, Sonogashira, and Heck cross-coupling reactions
- Copper-catalyzed nitration reactions
- Geometry relaxation-induced Large Stokes shift in red-emitting borondipyrromethenes (BODIPY) and applications in fluorescent thiol probes
Reagent used in Preparation of
- Photophysical properties of oxygen-containing polycyclic aromatic triptycenes
- Donor unit for donor-acceptor-type polymers via N-alkylation, Suzuki coupling, and bromination
- Aminopyridine-based inhibitors of mitotic kinase Nek2 with potential antipoliferative effects in cancer tumors
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Brett VanVeller et al.
Journal of the American Chemical Society, 134(17), 7282-7285 (2012-04-19)
The cyclization and planarization of polycyclic aromatic hydrocarbons with concomitant oxygen substitution was achieved through acid catalyzed transetherification and oxygen-radical reactions. The triptycene scaffold enforces proximity of the alcohol and arene reacting partners and confers significant rigidity to the resulting
Paolo Innocenti et al.
Journal of medicinal chemistry, 55(7), 3228-3241 (2012-03-13)
We report herein a series of Nek2 inhibitors based on an aminopyridine scaffold. These compounds have been designed by combining key elements of two previously discovered chemical series. Structure based design led to aminopyridine (R)-21, a potent and selective inhibitor
Man-Wah Tsang et al.
Biotechnology journal, 11(2), 257-265 (2015-08-08)
Rapid emergence of class C β-lactamases has urged an immediate need for developing class C β-lactamase specific inhibitors for effective clinical treatment. To facilitate the development of effective class C β-lactamase inhibitors, we propose a new approach for a rapid
Yinghui Chen et al.
The Journal of organic chemistry, 77(5), 2192-2206 (2012-02-10)
2-Thienyl and 2,6-bisthienyl BODIPY derivatives (BS-SS and BS-DS) were prepared that show intense absorption (ε = 65000 M(-1) cm(-1) at 507 nm) and a large Stokes shift (96 nm) vs the small Stokes shift of typical BODIPY (<15 nm). Control
Guo-Ping Lu et al.
The Journal of organic chemistry, 77(8), 3700-3703 (2012-04-07)
The ligands associated with various Pd catalysts play a crucial role in determining the stereochemistry of cross-couplings between boronic acids and Z-alkenyl halides. A ligand on palladium has been found that leads to the desired products under mild conditions and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
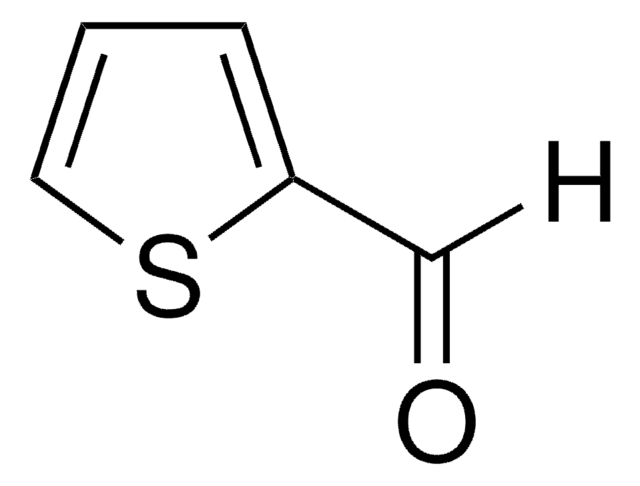
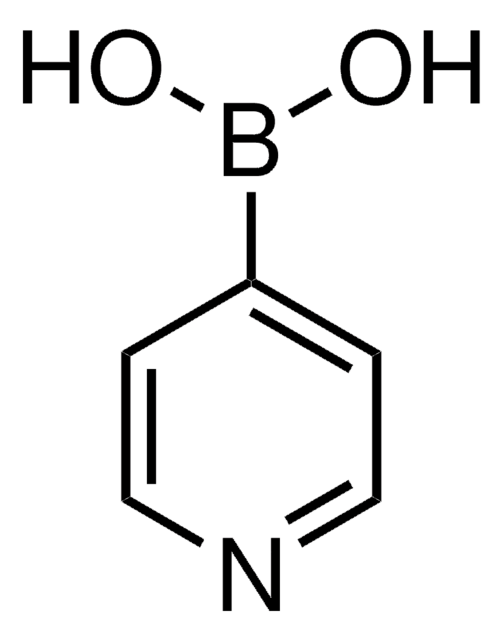

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)