All Photos(1)
About This Item
Empirical Formula (Hill Notation):
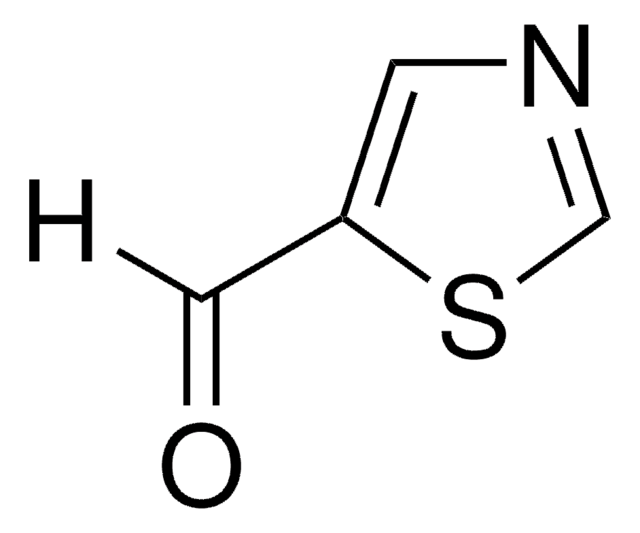
C4H3NOS
CAS Number:
Molecular Weight:
113.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.574 (lit.)
bp
61-63 °C/15 mmHg (lit.)
density
1.288 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O=Cc1nccs1
InChI
1S/C4H3NOS/c6-3-4-5-1-2-7-4/h1-3H
InChI key
ZGTFNNUASMWGTM-UHFFFAOYSA-N
General description
2-Thiazolecarboxaldehyde is a thiazole aldehyde derivative. It undergoes Baylis–Hillman reaction with methyl acrylate catalyzed by DABCO (1,4-diazabicyclo[2.2.2]octane). The reaction mechanism has been studied by electrospray ionization mass spectrometry (ESI-MS).
Application
2-Thiazolecarboxaldehyde may be used as a reactant in the following syntheses:
- Benzothiazine N-acylhydrazones, having potential antinociceptive and anti-inflammatory activity.
- Thiazole-2-yl-(amino)methylphosphonate diethyl esters.
- Imino ester by reacting with L-leucine t-butyl ester hydrochloride.
Useful building block for taxane analogs.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of new thiazole-2,-4, and-5-yl-(amino) methylphosphonates and phosphinates: unprecedented cleavage of thiazole-2 derivatives under acidic conditions.
Olszewski TK and Boduszek B.
Tetrahedron, 66(45), 8661-8866 (2010)
Yasuyuki Takeda et al.
Bioorganic & medicinal chemistry letters, 14(12), 3209-3215 (2004-05-20)
To improve the metabolic stability of 3, which exhibited both in vitro antitumor activity and in vivo efficacy by both iv and po administration, we designed and synthesized new taxane analogues. Most of the synthetic compounds maintained excellent antitumor activity
Online mechanistic investigations of catalyzed reactions by electrospray ionization mass spectrometry: a tool to intercept transient species in solution.
Santos LS.
European Journal of Organic Chemistry, 2008(2), 235-253 (2008)
The Morita-Baylis-Hillman Reaction: Advances and Contributions from Brazilian Chemistry.
Santos MS, et al.
Current Organic Synthesis, 12(6), 830-852 (2015)
Armel A Agbodjan et al.
The Journal of organic chemistry, 73(8), 3094-3102 (2008-03-25)
A practical asymmetric synthesis of a highly substituted N-acylpyrrolidine on multi-kilogram scale is described. The key step in the construction of the three stereocenters is a [3+2] cycloaddition of methyl acrylate and an imino ester prepared from l-leucine t-butyl ester
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service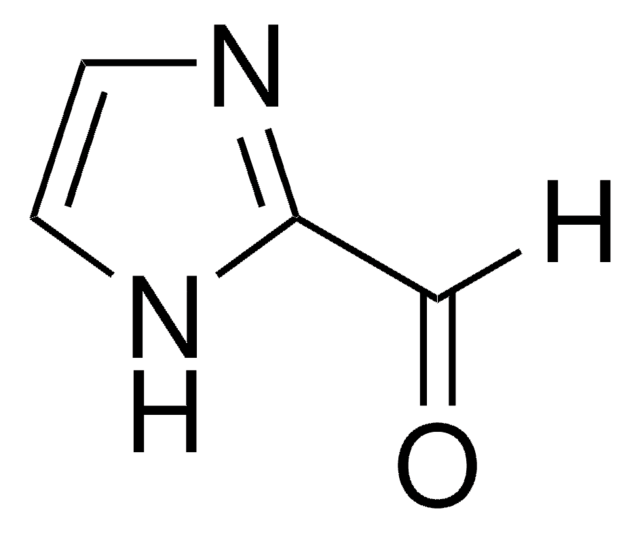
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)