389412
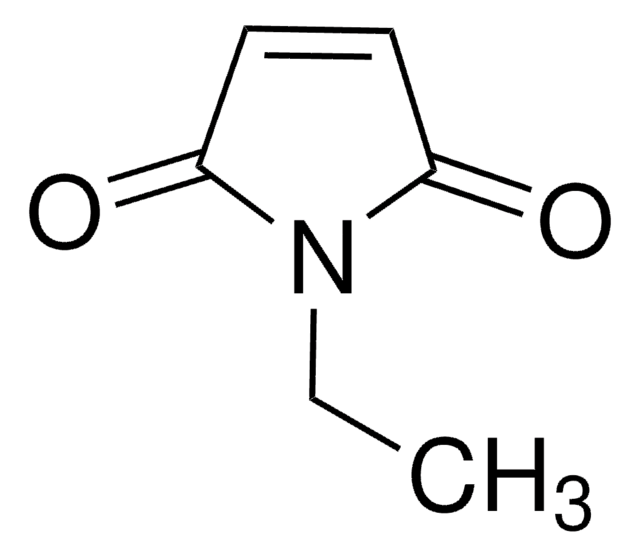
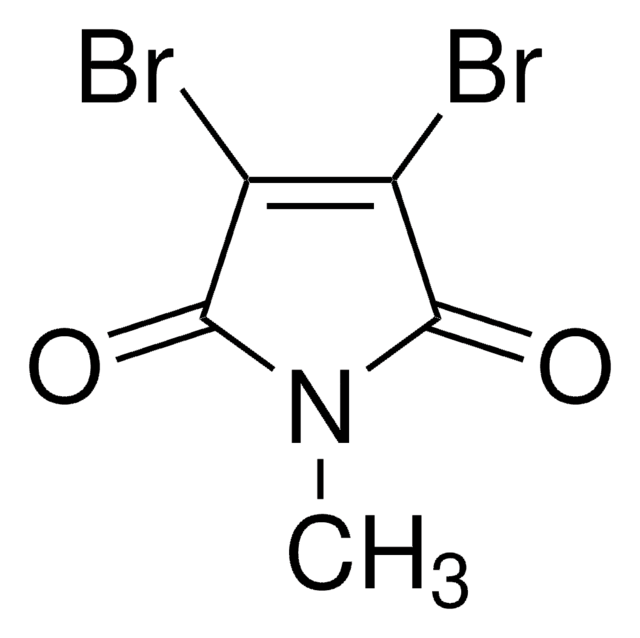
N-Methylmaleimide
97%
Synonym(s):
1-Methyl-1H-pyrrole-2,5-dione, 1-Methyl-2,5-dihydro-1H-pyrrole-2,5-dione, 1-Methylpyrrole-2,5-dione, N-Methyl maleic imide, N-Methylpyrrole-2,5-dione
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
mp
94-96 °C (lit.)
functional group
imide
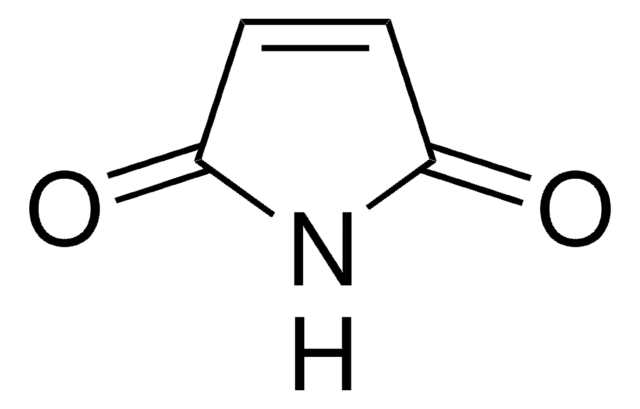
maleimide
SMILES string
CN1C(=O)C=CC1=O
InChI
1S/C5H5NO2/c1-6-4(7)2-3-5(6)8/h2-3H,1H3
InChI key
SEEYREPSKCQBBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In the synthesis of organic structure directing agents for designing silicogermanate zeolites.
- As a dienophile in Diels-Alder reaction.
- For the synthesis of biologically active pyrrolo[2,1-a]isoquinolines.
- As a dipolarophile in [3 + 2] dipolar addition reactions.
- As a co-monomer in atom transfer radical polymerization of styrene to obtain styrene copolymers with programmed microstructure.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service