363340
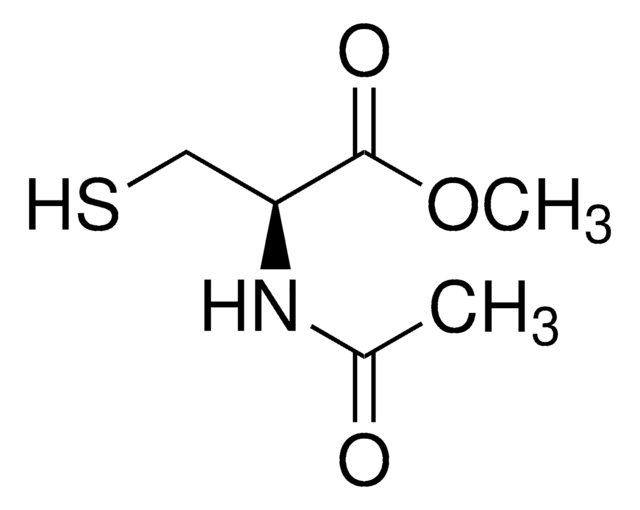
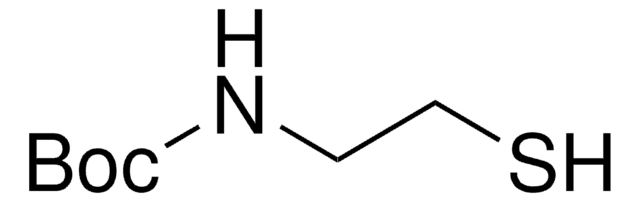
N-Acetylcysteamine
95%
Synonym(s):
N-(2-Mercaptoethyl)acetamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CONHCH2CH2SH
CAS Number:
Molecular Weight:
119.19
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
138-140 °C/7 mmHg (lit.)
mp
6-7 °C (lit.)
density
1.121 g/mL at 25 °C (lit.)
functional group
amide
thiol
SMILES string
CC(=O)NCCS
InChI
1S/C4H9NOS/c1-4(6)5-2-3-7/h7H,2-3H2,1H3,(H,5,6)
InChI key
AXFZADXWLMXITO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Acetylcysteamine, also known as N-(2-Mercaptoethyl) acetamide, is a derivative of cysteamine, that is commonly used as a building block for the synthesis of alkylated thiol and thioesters via esterification.
Application
N-Acetylcysteamine can be used as a building block to synthesize:
- N
- -acetylcysteamine (SNAC) thioesters by reacting with various acid derivatives in the presence of 1,1′-carbonyldiimidazole (CDI).
- Thieno[2,3-c]pyrrole derivatives via three-component reaction of 2-acetyl-3-thiophenecarboxaldehyde and various amines.
- Carbapenems, a class of beta-lactam antibiotic agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magoichi Sako et al.
The Journal of organic chemistry, 63(20), 6947-6951 (2001-10-24)
4H-[1,2,5]Oxadiazolo[3,4-d]pyrimidine-5,7-dione 1-oxides (2) are conveniently prepared in high yields by the oxidative intramolecular cyclization of 6-amino-5-nitro-1H-pyrimidine-2,4-diones (1) employing iodosylbenzene diacetate as an oxidant in the presence of lithium hydride. The generation of nitric oxide (NO) and NO-related species from 2
Isolation, properties, and regulation of a mitochondrial acyl coenzyme A thioesterase from pig heart.
K Y Lee et al.
The Journal of biological chemistry, 254(11), 4516-4523 (1979-06-10)
N Singh et al.
Biochemical and biophysical research communications, 131(2), 786-792 (1985-09-16)
The acetyl transacylase activity of the fatty acid synthase from yeast has been investigated using p-nitrophenylthiol acetate. The chromophoric nature of the nitrophenylthiol moiety affords a convenient spectrophotometric assay for the transacylase function as well as a means to investigate
Tetrahedron Letters, 29, 4305-4305 (1988)
Thomas Frenzel et al.
Organic letters, 8(1), 135-138 (2005-12-31)
[structure: see text] The enantioselective total synthesis of the N-acetylcysteamine thioester of seco-proansamitocin, a key biosynthetic intermediate of the highly potent antitumor agent ansamitocin, is described, which twice utilizes the Nagao acetate aldol reaction, as well as an indium-mediated alkynylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service