All Photos(1)
About This Item
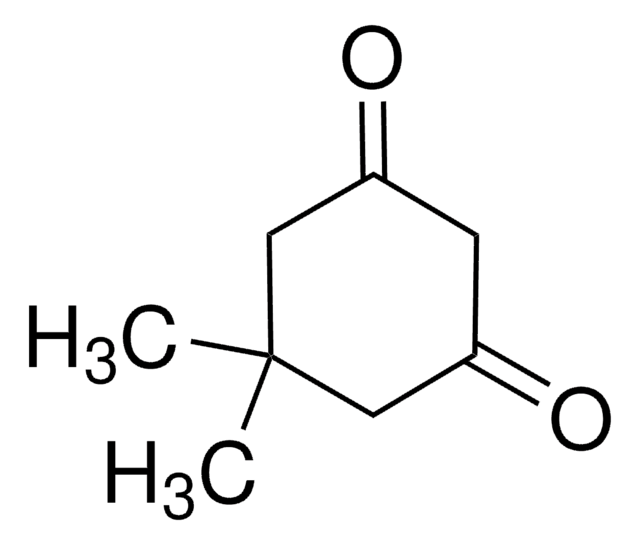
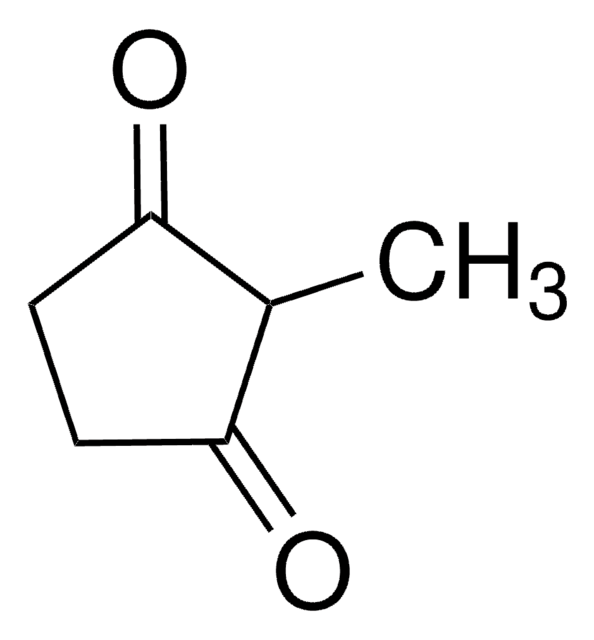
Linear Formula:
(CH3)2C6H6(=O)2
CAS Number:
Molecular Weight:
140.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
130 °C/7 mmHg (lit.)
mp
103-105 °C (lit.)
functional group
ketone
SMILES string
CC1(C)CCC(=O)CC1=O
InChI
1S/C8H12O2/c1-8(2)4-3-6(9)5-7(8)10/h3-5H2,1-2H3
InChI key
PLGPBTCNKJQJHQ-UHFFFAOYSA-N
Related Categories
General description
Standard molar enthalpy of formation of 4,4-dimethyl-1,3-cyclohexanedione in the gaseous state at 298.15K has been determined.
Application
4,4-Dimethyl-1,3-cyclohexanedione was employed in the synthesis of series of 4-aryl-6,6-dimethyl-1,2,3,4,5,6,7,8- octahydroquinazoline-2,5-diones via Biginelli reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Saraç et al.
Die Pharmazie, 56(4), 298-302 (2001-05-08)
In this study, a series of 4-aryl-6,6-dimethyl-1,2,3,4,5,6,7,8- octahydroquinazoline-2,5-diones were synthesized by condensing urea with 4,4-dimethyl-1,3-cyclohexanedione and appropriate aromatic aldehydes according to the Biginelli reaction. The structures of the compounds were characterized by spectroscopic methods. The racemic compounds were resolved into
Thermochemical and theoretical studies on cyclohexanediones.
Pilcher G, et al.
The Journal of Physical Chemistry, 97(1), 243-247 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service