301590
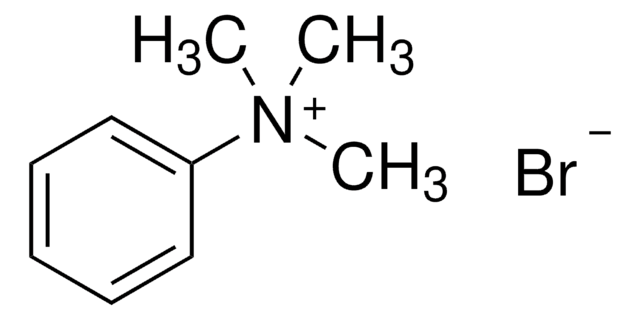
Tetrabutylammonium tribromide
98%
Synonym(s):
TBABr3, Tetrabutylammonium bromide perbromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4NBr3
CAS Number:
Molecular Weight:
482.18
Beilstein:
3746114
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
71-76 °C (lit.)
SMILES string
Br[Br-]Br.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.Br3/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-3-2/h5-16H2,1-4H3;/q+1;-1
InChI key
XXSLZJZUSYNITM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Tetrabutylammonium tribromide can be used:
- As a catalyst in the preparation of O-isopropylidene derivatives of free sugars.
- As a reagent in methanol for the cleavage of tert-butyldimethylsilyl (TBDMS) ethers chemoselectively.
- As a catalyst in the synthesis of bis-indolymethanes by electrophilic substitution of indoles with different aldehydes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrabutylammonium tribromide (TBATB): a mild and efficient catalyst for O-isopropylidenation of carbohydrates
Khan AT, et al.
Carbohydrate Research, 346(5), 673-677 (2011)
Tetrabutylammonium tribromide (TBATB)- MeOH: An efficient chemoselective reagent for the cleavage of tert-butyldimethylsilyl (TBDMS) ethers
Gopinath R and Patel BK
Organic Letters, 2(26), 4177-4180 (2000)
Mild and Efficient Synthesis of bis-Indolylmethanes Catalyzed by Tetrabutylammonium Tribromide
Lin X, et al.
Synthetic Communications, 36(21), 3153-3160 (2006)
Shigeo Hayashi et al.
Journal of enzyme inhibition and medicinal chemistry, 29(6), 846-867 (2014-02-13)
Because of the pivotal role of cyclooxygenase (COX) in the inflammatory processes, non-steroidal anti-inflammatory drugs (NSAIDs) that suppress COX activities have been used clinically for the treatment of inflammatory diseases/syndromes; however, traditional NSAIDs exhibit serious side-effects such as gastrointestinal damage
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 301590-5G | |
| 301590-100G | 4061826665480 |
| 301590-25G | 4061826665497 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service