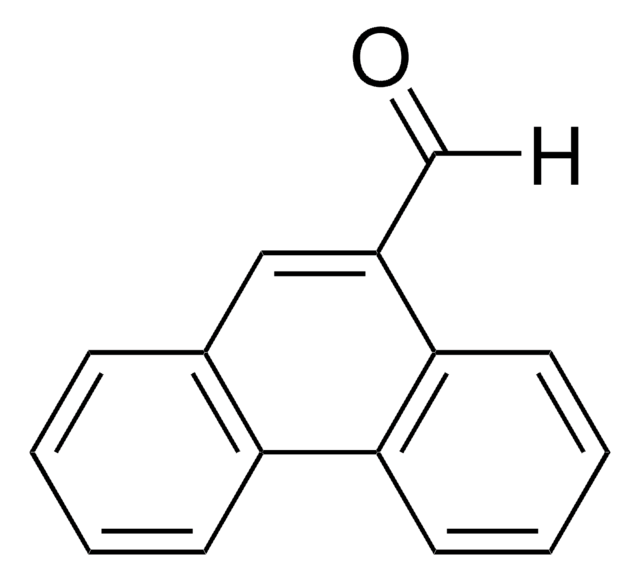
278688
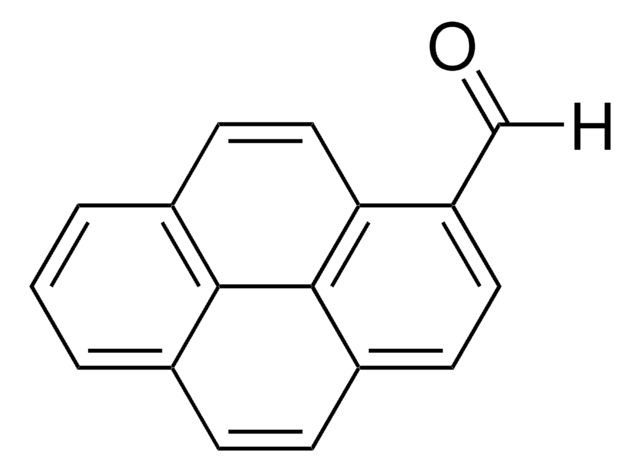
9-Anthracenecarboxaldehyde
97%
Synonym(s):
9-Anthraldehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C15H10O
CAS Number:
Molecular Weight:
206.24
Beilstein:
639167
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
103-105 °C (lit.)
SMILES string
[H]C(=O)c1c2ccccc2cc3ccccc13
InChI
1S/C15H10O/c16-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)15/h1-10H
InChI key
YMNKUHIVVMFOFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The Diels-Alder reaction of 9-anthracenecarboxaldehyde with benzenediazonium-2-carboxylate was studied.
Application
9-Anthracenecarboxaldehyde has been used in the synthesis of:
- new asymmetrical tridentate Schiff base ligands
- 2-(9-anthrylmethyl-ideneamino)-4-methyl-phenol, novel Schiff base via condensation with 2-amino-p-cresol
- functionalized ligand, 2-(anthracen-9-ylidene)-4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione via Knoevenangel condensation with the diphosphine ligand 4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Knoevenagel condensation of the diphosphine ligand 4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione with 9-anthracenecarboxaldehyde: Synthesis of the second-generation ligand 2-(anthracen-9-ylidene)-4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione.
Watson WH, et al.
Journal of Chemical Crystallography, 36(11), 715-722 (2006)
Biswonath Biswal et al.
Dalton transactions (Cambridge, England : 2003), 46(28), 8975-8991 (2017-06-27)
A tri-fluorophoric molecular probe (1) with three different derivatized fluorophores, i.e. anthracene (An), 7-nitrobenz-2-oxa-1,3-diazole (NBD) and rhodamine-B (Rh) appended on to a Tren [tris-(2-aminoethyl)amine] receptor was demonstrated to exhibit metal ion induced ratiometric fluorescence signalling through the initiation of a
Skrollan Stockinger et al.
Beilstein journal of organic chemistry, 9, 1837-1842 (2013-09-26)
A new approach for the investigation of a higher-order reaction by on-column reaction gas chromatography is presented. The reaction and the analytical separation are combined in a single experiment to investigate the Diels-Alder reaction of benzenediazonium-2-carboxylate as a benzyne precursor
Mustafa Şahin et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 103, 400-408 (2013-01-01)
New asymmetrical tridentate Schiff base ligands were synthesized using 1,2-phenylenediamine, 4-methyl-1,2-phenylenediamine, 2-hydroxy-1-napthaldehyde, 9-anthracenecarboxaldehyde. Schiff base ligands and their metal complexes were synthesised and characterized by using FT-IR, (1)H NMR, (13)C NMR, UV-Vis, XRD, ESR, elemental analysis and fluorescence studies. The
Andrés Villalpando et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 6), o1353-o1353 (2010-01-01)
The title compound, C(22)H(17)NO, is a novel Schiff base synthesized via a condensation reaction between 9-anthracenecarboxaldehyde and 2-amino-p-cresol. The asymmetric unit contains two independent mol-ecules that are joined by an O-H⋯OH hydrogen bond. An intra-molecular O-H⋯N hydrogen bond occurs in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service