244325
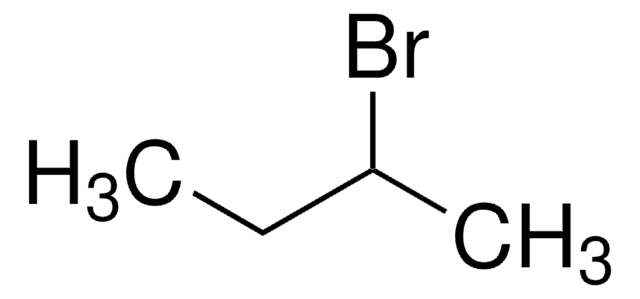
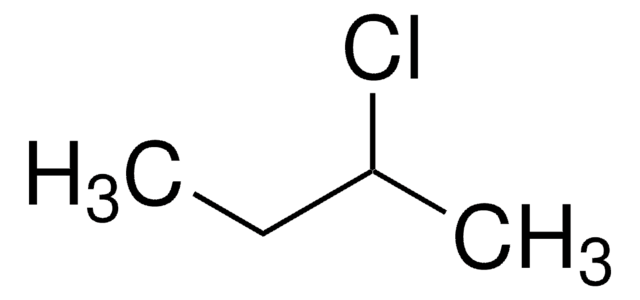
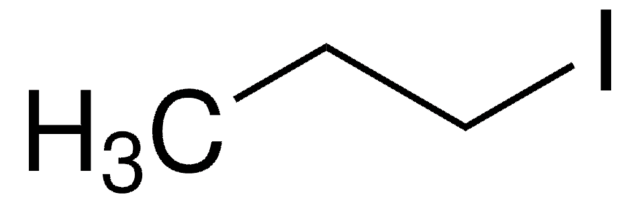
2-Iodobutane
≥98%, contains copper as stabilizer
Synonym(s):
sec-Butyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
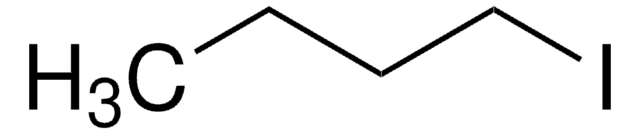
CH3CH2CHICH3
CAS Number:
Molecular Weight:
184.02
Beilstein:
1718777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.499 (lit.)
bp
119-120 °C (lit.)
mp
−104 °C (lit.)
solubility
alcohol: soluble(lit.)
diethyl ether: soluble(lit.)
water: insoluble(lit.)
density
1.598 g/mL at 25 °C (lit.)
functional group
alkyl halide
iodo
SMILES string
CCC(C)I
InChI
1S/C4H9I/c1-3-4(2)5/h4H,3H2,1-2H3
InChI key
IQRUSQUYPCHEKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Iodobutane was degraded by reductive dehalogenation with nickel-aluminum alloy in potassium hydroxide solution. The release rate of 2-iodobutane, a volatile organoiodide, was studied.
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
75.2 °F - closed cup
Flash Point(C)
24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Frank Keppler et al.
Chemosphere, 52(2), 477-483 (2003-05-10)
Volatile iodinated organic compounds play an important role in the tropospheric photochemical system, but the current knowledge of the known sources and sinks of these alkyl iodides is still incomplete. This paper describes a new source of alkyl iodides from
Ying Wang et al.
Nature communications, 10(1), 5395-5395 (2019-12-05)
Synthesis of higher carboxylic acids using CO2 and H2 is of great importance, because CO2 is an attractive renewable C1 resource and H2 is a cheap and clean reductant. Herein we report a route to produce higher carboxylic acids via
G Lunn et al.
American Industrial Hygiene Association journal, 52(6), 252-257 (1991-06-01)
Two techniques were investigated for degrading a number of halogenated compounds of commercial and research importance. Reductive dehalogenation with nickel-aluminum alloy in potassium hydroxide solution was used to degrade iodomethane, chloroacetic acid, trichloroacetic acid, 2-chloroethanol, 2-bromoethanol, 2-chloroethylamine, 2-bromoethylamine, 1-bromobutane, 1-iodobutane
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 244325-500G | |
| 244325-100G | 4061825751795 |
| 244325-5G | 4061825751801 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service