240230
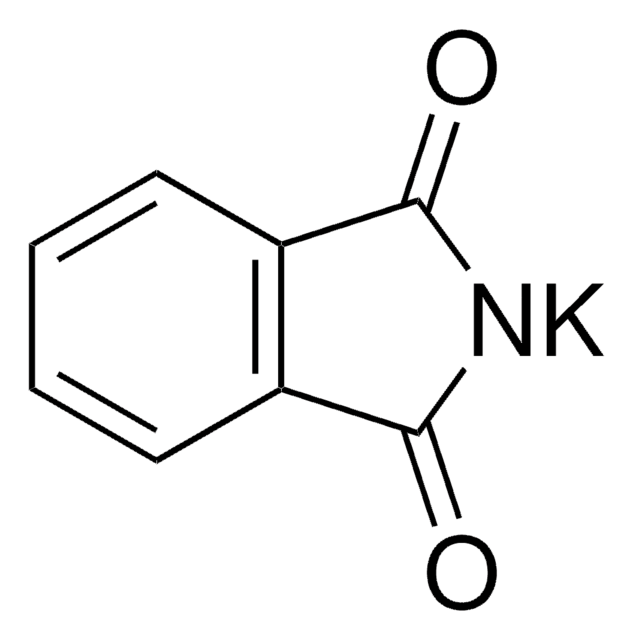
Phthalimide
≥99%
Synonym(s):
1,3-Dihydro-1,3-dioxoisoindole
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C8H5NO2
CAS Number:
Molecular Weight:
147.13
Beilstein:
118522
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
mp
232-235 °C (lit.)
solubility
water: slightly soluble(lit.)
SMILES string
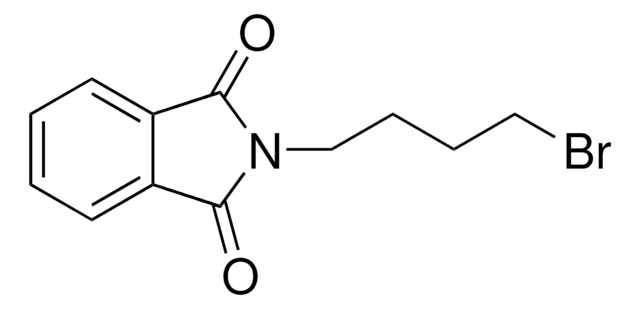
O=C1NC(=O)c2ccccc12
InChI
1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)8(11)9-7/h1-4H,(H,9,10,11)
InChI key
XKJCHHZQLQNZHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Phthalimide was used in the synthesis of 6-amino agarose (AA) by Mitsunobu-inspired microwave mediated method.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stalin Kondaveeti et al.
Carbohydrate polymers, 106, 365-373 (2014-04-12)
A facile 6-aminoagarose (AA) mediated synthesis of new fluorogenic amides of agarose with nicotinic (AA-NA) and picolinic acids (AA-PA) employing carbodiimide chemistry have been described. 6-Amino agarose (AA) was synthesized in a facile Mitsunobu-inspired microwave mediated method involving the reaction
Alessio Pierini et al.
Journal of veterinary science, 21(5), e60-e60 (2020-10-06)
Tumor-associated neoangiogenesis is a crucial target for antitumor therapies. Thalidomide (TAL) is a promising anti-neoangiogenetic drug that has recently been used in the treatment of several malignancies in dogs. The aim of the study was to assess the pharmacokinetics of
Shi Tang et al.
Organic letters, 10(6), 1179-1182 (2008-02-23)
A novel palladium-catalyzed intermolecular aminopalladation/C-H activation method for selectively synthesizing (E)-(2-oxindolin-3-ylidene)phthalimides has been developed. In the presence of Pd(OAc)2 and PhI(OAc)2, alkynes were difunctionalized with a phthalimide and an arene sp2 C-H bond to selectively synthesize (E)-(2-oxoindolin-3-ylidene)phthalimides, which products are
Barbara Weiner et al.
Journal of the American Chemical Society, 131(27), 9473-9474 (2009-07-09)
A new method for the synthesis of beta(3)-amino acids is presented. Phthalimide protected allylic amines are oxidized under Wacker conditions selectively to aldehydes using PdCl(2) and CuCl or Pd(MeCN)(2)Cl(NO(2)) and CuCl(2) as complementary catalyst systems. The aldehydes are produced in
Edward C Lawson et al.
Journal of medicinal chemistry, 52(23), 7432-7445 (2009-09-08)
We have discovered two related chemical series of nonpeptide urotensin-II (U-II) receptor antagonists based on piperazino-phthalimide (5 and 6) and piperazino-isoindolinone (7) scaffolds. These structure types are distinctive from those of U-II receptor antagonist series reported in the literature. Antagonist
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service