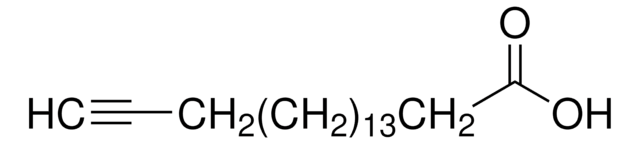232211
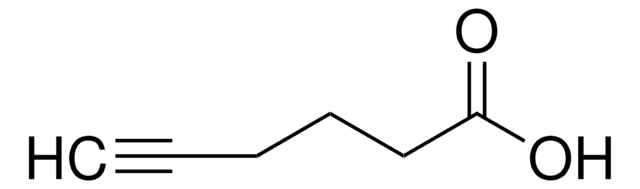
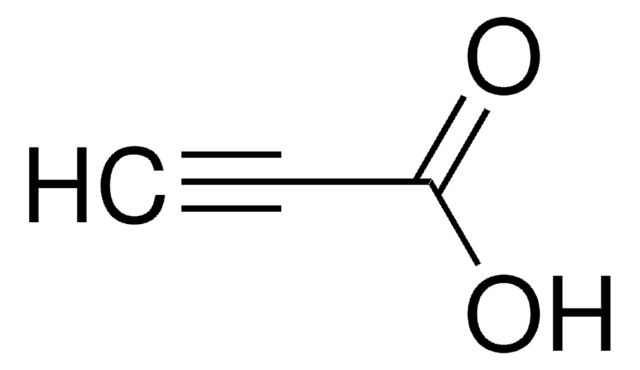
4-Pentynoic acid
95%
Synonym(s):
Propargylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH≡CCH2CH2COOH
CAS Number:
Molecular Weight:
98.10
Beilstein:
1742047
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI key
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Pentynoic acid undergoes copper-catalyzed intramolecular cyclizations to form enol lactones. It also reacts with 1-bromo-1-alkynes in the presence of a Pd catalyst to yield biologically active ynenol lactones.
Application
4-Pentynoic acid was used:
- as building block for the synthesis of library of eight sequence-defined model oligomers
- in one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives
- in the synthesis of various allenenols lactones [5(E)-(2-allenylidene)-tetrahydro-2-furanones]
- in the synthesis of a cyctotoxic macrolide by ring-closing metathesis of a bis acetylene
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium-catalyzed synthesis of new unsaturated exo-enol lactones with potential biological activity.
Bouyssi D, et al.
Tetrahedron Letters, 34(19), 3129-3130 (1993)
Xin Zhou et al.
Acta biomaterialia, 54, 128-137 (2017-03-13)
Wound healing dressings are increasingly needed clinically due to the large number of skin damage annually. Nitric oxide (NO) plays a key role in promoting wound healing, thus biomaterials with NO-releasing property receive increasing attention as ideal wound dressing. In
Thomas L Mindt et al.
The Journal of organic chemistry, 72(26), 10247-10250 (2007-11-30)
Alkynoic acids, in particular, 4-pentynoic acid derivatives, undergo intramolecular cyclizations to enol lactones under reaction conditions typically applied for the Cu(I)-catalyzed cycloaddition of terminal alkynes and azides (click chemistry). Starting from appropriate alkynoic acid derivatives, either enol lactones or 1,2,3-triazole
Tetrahedron Letters, 33, 2811-2811 (1992)
Xun Ji et al.
The Journal of organic chemistry, 78(9), 4312-4318 (2013-04-06)
An efficient and facile Au(I)/Ag(I)-catalyzed cascade method has been developed for one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives through treatment of the substituted 2-(1H-benzo[d]imidazol-2-yl)anilines with 4-pentynoic acid or 5-hexynoic acid. The strategy features a Au(I)/Ag(I)-catalyzed one-pot cascade process
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service