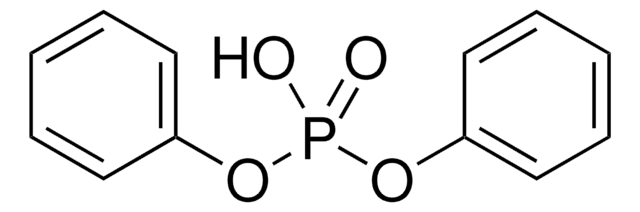
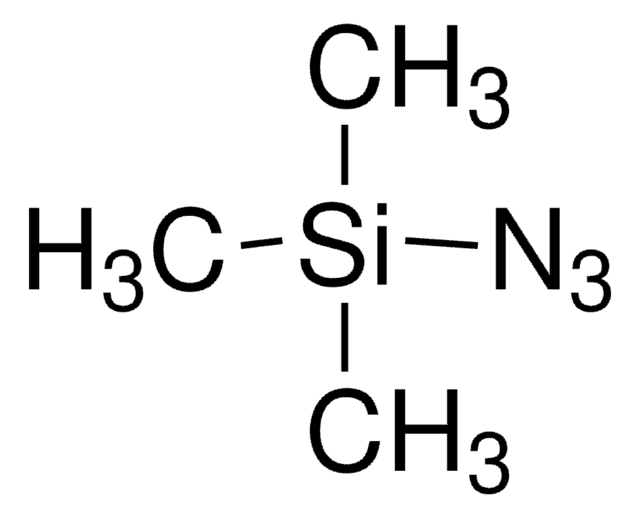178756
Diphenyl phosphoryl azide
97%
Synonym(s):
DPPA, Phosphoric acid diphenyl ester azide
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.551 (lit.)
bp
157 °C/0.17 mmHg (lit.)
density
1.277 g/mL at 25 °C (lit.)
functional group
azide
storage temp.
2-8°C
SMILES string
[N-]=[N+]=NP(=O)(Oc1ccccc1)Oc2ccccc2
InChI
1S/C12H10N3O3P/c13-14-15-19(16,17-11-7-3-1-4-8-11)18-12-9-5-2-6-10-12/h1-10H
InChI key
SORGEQQSQGNZFI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent for synthesis of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)