134775
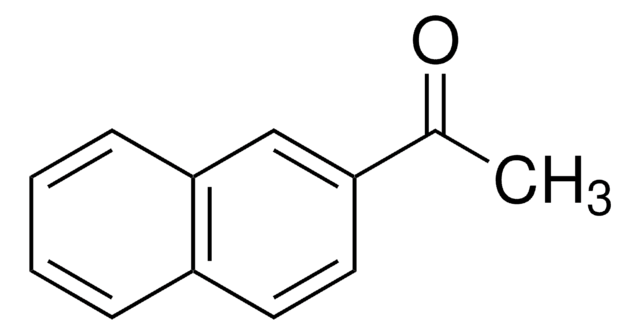
2-Acetonaphthone
99%
Synonym(s):
2′-Acetonaphthone, 2-Acetylnaphthalene, Methyl 2-naphthyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H7COCH3
CAS Number:
Molecular Weight:
170.21
Beilstein:
774965
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
300-301 °C (lit.)
mp
52-56 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1ccc2ccccc2c1
InChI
1S/C12H10O/c1-9(13)11-7-6-10-4-2-3-5-12(10)8-11/h2-8H,1H3
InChI key
XSAYZAUNJMRRIR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Acetonaphthone undergoes efficient photoreduction in the presence of tri-n-butylstannane as hydrogen donor. It is solubilized in air-saturated sodium dodecyl sulphate micelles in D2O or H2O by pulsed nitrogen laser photolysis for triplet sensitized production of singlet oxygen.
Application
2-Acetonaphthone was used in direct time-resolved studies on singlet molecular oxygen phosphorescence in heterogeneous silica gel/cyclohexane systems.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
334.4 °F - DIN 51758
Flash Point(C)
168 °C - DIN 51758
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mechanisms of Photoreactions in Solution. VI. 1 Reduction of 1-Naphthaldehyde and 2-Acetonaphthone.
Hammond GS and Leermakers PA.
Journal of the American Chemical Society, 84(2), 207-211 (1962)
Lifetime and reactivity of singlet oxygen in an aqueous micellar system: a pulsed nitrogen laser study.
Gorman AA and Rodgers MAJ.
Chemical Physics Letters, 55(1), 52-54 (1978)
Quenching of singlet molecular oxygen (1. DELTA. gO2) in silica gel/cyclohexane heterogeneous systems. A direct time-resolved study.
Iu KK and Thomas JK.
Journal of the American Chemical Society, 112(9), 3319-3325 (1990)
Russell J Chedgy et al.
Phytochemistry, 113, 149-159 (2015-01-07)
Salicinoids are phenolic glycosides (PGs) characteristic of the Salicaceae and are known defenses against insect herbivory. Common examples are salicin, salicortin, tremuloidin, and tremulacin, which accumulate to high concentrations in the leaves and bark of willows and poplars. Although their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service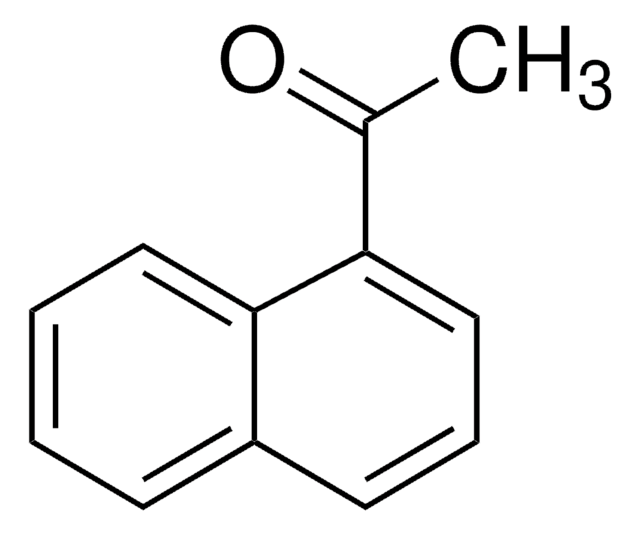
![4-(1′,5′-Dihydro-1′-methyl-2′H-[5,6]fullereno-C60-Ih-[1,9-c]pyrrol-2′-yl)benzoic acid](/deepweb/assets/sigmaaldrich/product/structures/417/736/540e4dd8-0c87-48e5-8307-3befb16498ba/640/540e4dd8-0c87-48e5-8307-3befb16498ba.png)