1224981
USP
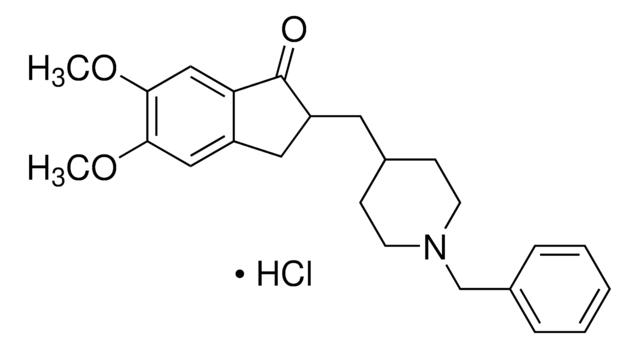
Donepezil hydrochloride
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
(±)-2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-1-indanone hydrochloride, 2,3-Dihydro-5,6-dimethoxy-2-{[1-(phenylmethyl)-4-piperidinyl]methyl}-1H-inden-1-one
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
donepezil
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
InChI
1S/C24H29NO3.ClH/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18;/h3-7,14-15,17,20H,8-13,16H2,1-2H3;1H
InChI key
XWAIAVWHZJNZQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Novel tacrine-donepezil hybrids for Alzheimer′s treatment: Research has led to the development of new tacrine-donepezil hybrid compounds targeting Alzheimer′s disease, offering enhanced bioactivity and potential therapeutic benefits. These hybrids demonstrate multifunctional approaches in addressing neurodegenerative disorders by combining the acetylcholinesterase inhibitory effects of donepezil with the neuroprotective properties of tacrine (Bayraktar et al., 2024).
- Role of oligodendrocytes in Alzheimer′s disease: A study explores the involvement of oligodendrocytes in Alzheimer′s pathogenesis, highlighting potential mechanisms where donepezil could be implicated in modulating neurodegenerative processes. This research underscores the broader applicability of donepezil in understanding and potentially treating Alzheimer′s disease (Wang et al., 2024).
- Shenghui decoction′s neuroprotective effects: Investigating traditional therapies, this study assesses the neuroprotective effects of Shenghui decoction in an Alzheimer′s disease model. Donepezil′s role in this context is compared, contributing to broader insights into potential integrative treatments for neurodegenerative diseases (Lu et al., 2024).
- Electrochemical sensors for Alzheimer′s drug analysis: The development of electrochemical acetylcholinesterase sensors designed for the detection and study of anti-Alzheimer drugs like donepezil underscores its importance in research and therapeutic monitoring. This advancement supports the precise administration and study of donepezil in clinical settings (Ivanov et al., 2024).
- Modulating neurodegeneration with donepezil: A study on the combined effects of endothelin receptor agonist IRL-1620 with donepezil in an amyloid-β induced model of neurodegeneration in rats, expands our understanding of donepezil′s therapeutic potential beyond traditional applications, suggesting its utility in complex therapeutic regimes (Mahajan et al., 2024).
Analysis Note
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Eye Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
1224981-200MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service