575656-U
HybridSPE®-Phospholipid 96-well solid phase extraction (SPE) Plate
Volume 2 mL, bed wt., 50 mg, pk of 1
Synonym(s):
HybridSPE (phospholipid and protein removal) 96-well plate, 50 mg
About This Item
Recommended Products
product name
HybridSPE®-Phospholipid, 96-well Plate, bed wt. 50 mg, volume 2 mL, pk of 1
product line
HybridSPE®
form
solid
composition
bed wt., 50 mg
packaging
pk of 1
technique(s)
solid phase extraction (SPE): suitable
volume
2 mL
matrix active group
zirconia-based phase
Looking for similar products? Visit Product Comparison Guide
General description
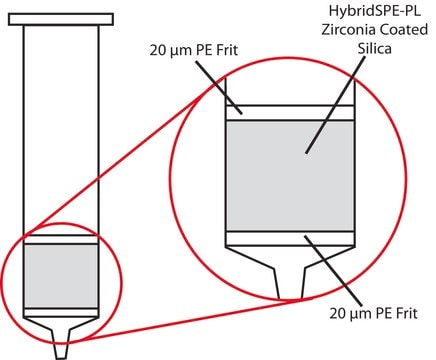
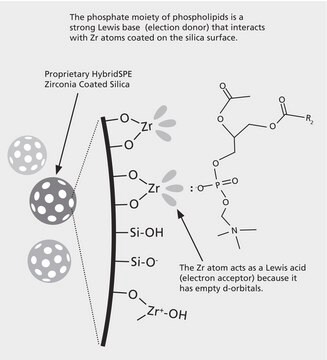
The "In-well" and "In-cartridge" precipitation methods are available for the HybridSPE-Phospholipid 96-well version and HybridSPE-Phospholipid Ultra cartridge in which biological plasma/serum is first added to either the well or cartridge, followed by acidified acetonitrile (precipitation agent). After a brief mixing/vortexing step, vacuum is applied. Because the 96-well and Ultra cartridge versions contain a series of low porosity hydrophobic filters/frits, the packed-bed filter/frit assembly acts as a depth filter facilitating the concurrent removal of both phospholipids and precipitated proteins during the extraction process. Standard HybridSPE-Phospholipid cartridges require an "off-line" precipitation method.
Application
- Rapid analysis of 65 pharmaceuticals and 7 personal care products in plasma and whole-body tissue samples of fish using acidic extraction, zirconia-coated silica cleanup, and liquid chromatography-tandem mass spectrometry: This study discusses the advanced application of chromatography and mass spectrometry, which could involve the use of technologies like HybridSPE®-Phospholipid for sample preparation, specifically for rapid analysis in biological matrices (Tanoue et al., 2020).
- Multi LC-MS/MS and LC-HRMS Methods for Determination of 24 Mycotoxins including Major Phase I and II Biomarker Metabolites in Biological Matrices from Pigs and Broiler Chickens: Demonstrates how LC-MS/MS, potentially incorporating HybridSPE®-Phospholipid technology for sample cleanup, facilitates the sensitive and accurate detection of mycotoxins in complex biological samples (Lauwers et al., 2019).
- Evaluation of an Ion Trap Toxtyper Liquid Chromatography With An Ion Trap Mass Spectrometric Instrument (Toxtyper) for Drug of Abuse Screening in Oral Fluid: Explores the utility of specific LC-MS/MS methodologies which could integrate HybridSPE®-Phospholipid for bioanalytical sample cleanup, enhancing drug screening accuracy (Plecko et al., 2018).
- Effective phospholipid removal from plasma samples by solid phase extraction with the use of copper (II) modified silica gel cartridges: This research potentially aligns with the use of HybridSPE®-Phospholipid technologies for phospholipid removal, focusing on enhancing the purity and quality of chromatography samples (Flieger et al., 2017).
- Liquid chromatography mass spectrometry determination of perfluoroalkyl acids in environmental solid extracts after phospholipid removal and on-line turbulent flow chromatography purification: Illustrates a specialized application of chromatography and mass spectrometry where HybridSPE®-Phospholipid could be applied to improve the analysis of environmental samples by efficient phospholipid removal (Mazzoni et al., 2016).
Features and Benefits
- Merges the simplicity of protein precipitation and the selectivity of SPE via the targeted removal of phospholipids
- Reduce ion-suppression through the complete removal of phospholipids and precipitated proteins
- 2-3 step generic procedure
- Minimal to no method development
- Available in 96-well and 1 mL cartridge dimensions
Legal Information
Application
SPE tube or plate
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
575656-U:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
This study demonstrates the impact of various mobile phase modifiers on the separation; formate modifiers outperform acetate in terms of MS signals (or sensitivity) and chromatographic resolution.
We are presenting an article focusing on ion-suppression and phospholipid contamination and some of their major causes and difficulties.
This Sigma-Aldrich article discusses how the HybridSPE-Phospholipid Technology works and how the phospholipids are removed.
Improvement in LC/MS Analysis of Vitamin D Metabolites in Serum by Leveraging Column Selectivity and Effective Sample Prep
Protocols
his study demonstrates the analysis of Warfarin in plasma samples utilizing chiral and achiral (reversed-phase) LC-MS and effective sample prep to remove endogenous phospholipids
A simple method to enrich phospholipids from plasma samples, involving a HybridSPE-PPT 96-well plate that both retains phospholipids and removes precipitated proteins.
The conversion of clinical methods to LC/MS/MS offers advantages; however, is accompanied by a few limitations, notably interference effects from the endogenous sample matrix.
Chromatograms
application for HPLCapplication for HPLCapplication for SPE, application for LC-MSapplication for HPLCShow MoreOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service