442505
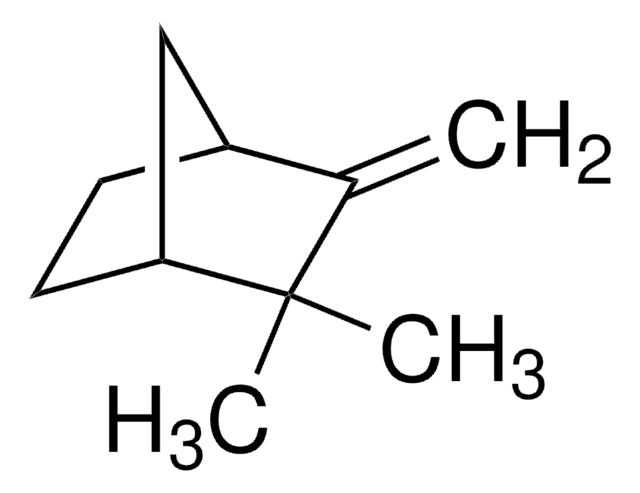
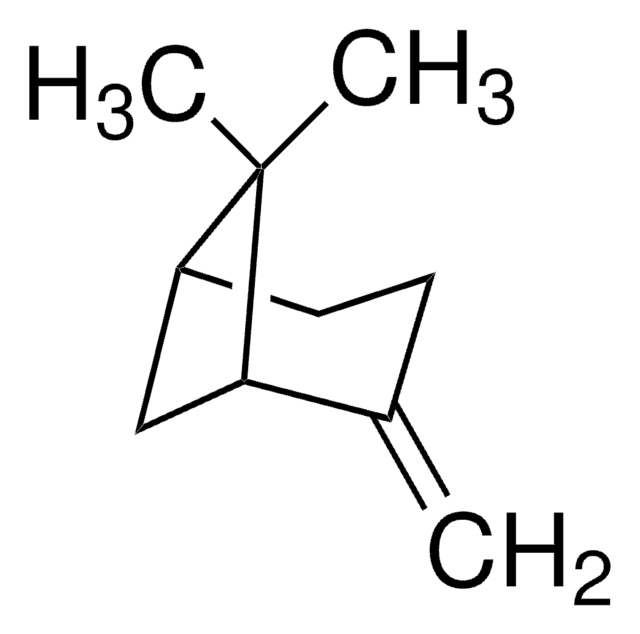
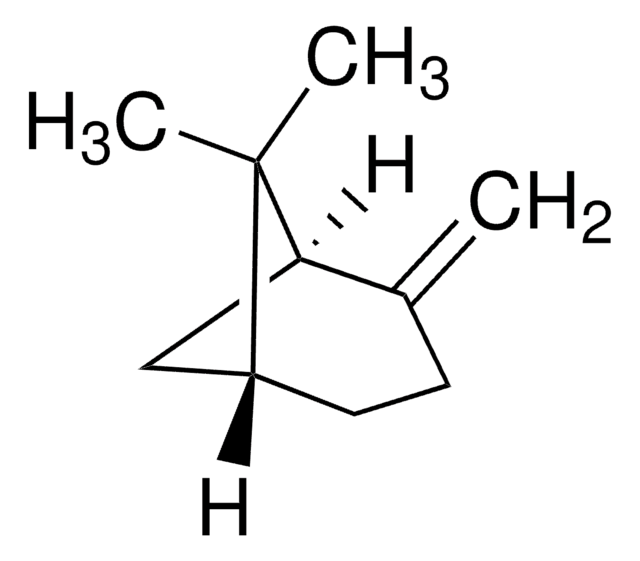
Camphene
analytical standard
Synonym(s):
DL-Camphene
About This Item
Recommended Products
grade
analytical standard
Quality Level
CofA
current certificate can be downloaded
packaging
ampule of 1000 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
159-160 °C (lit.)
mp
48-52 °C (lit.)
density
0.85 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
2-30°C
SMILES string
[H][C@]12CC[C@]([H])(C1)C(C)(C)C2=C
InChI
1S/C10H16/c1-7-8-4-5-9(6-8)10(7,2)3/h8-9H,1,4-6H2,2-3H3/t8-,9+/m0/s1
InChI key
CRPUJAZIXJMDBK-DTWKUNHWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 2
Flash Point(F)
78.8 °F - DIN 51755 Part 1
Flash Point(C)
26 °C - DIN 51755 Part 1
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 2: Flammable solids
Flammable solid
Hazardous rank III
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
442505:
442505-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service