SRE0045
Luciferase from Photinus pyralis (firefly)
recombinant, expressed in E. coli, lyophilized powder, ≥10×1010 units/mg protein
Synonym(s):
Luciferase firefly
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
lyophilized powder
specific activity
≥10×1010 units/mg protein
mol wt
62 kDa
application(s)
diagnostic assay manufacturing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
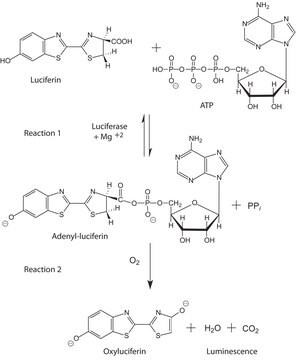
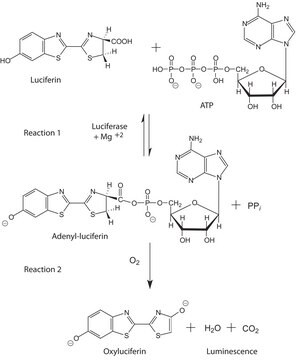
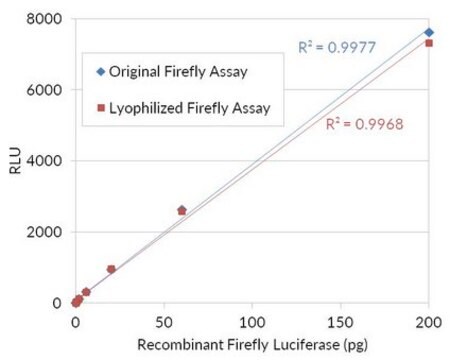
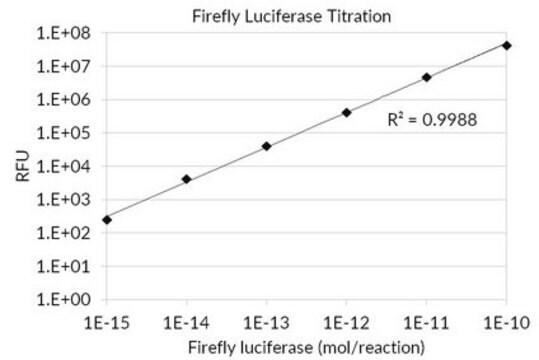
Unit Definition
Unit Definition Conversion Factor: There are approximately 9000 Relative Light Units (RLU) per one traditional Light Unit that uses a peak height equivalent to 0.02 μCi of 14C in a PPO/POPOP cocktail.
Physical form
Preparation Note
After reconstitution, the enzyme solutions can kept at 4-8 °C for up to 2 days or frozen in working aliquots at -20°C for at least one month. Repeated freezing and thawing is not recommended.
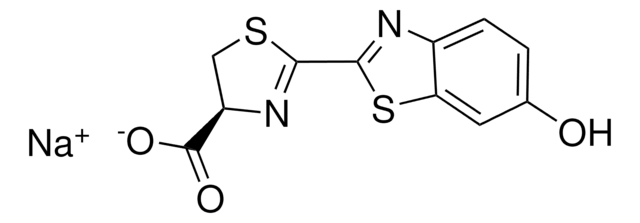
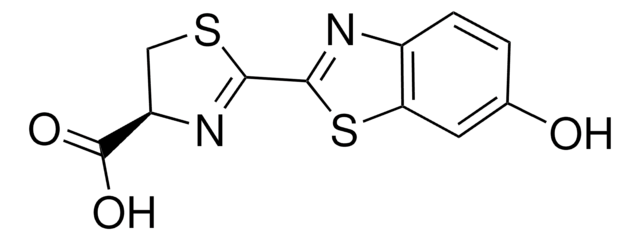
substrate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
JAN Code
SRE0045-2MG-PW:
SRE0045-1MG-PW:
SRE0045-VAR:
SRE0045-1MG:
SRE0045-BULK:
SRE0045-10MG-PW:
SRE0045-10MG:
SRE0045-2MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Firefly luciferase is a widely used bioluminescent reporter for studying gene regulation and function. It is a very sensitive genetic reporter due to the absence of endogenous luciferase activity in mammalian cells or tissues.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service