RAB0743
Human HAVCR1 / Hepatitis A Virus Cellular Receptor 1 ELISA Kit
About This Item
Recommended Products
species reactivity
human
packaging
kit of 96 wells (12 strips x 8 wells)
technique(s)
ELISA: suitable
input
sample type plasma
sample type cell culture supernatant(s)
sample type serum
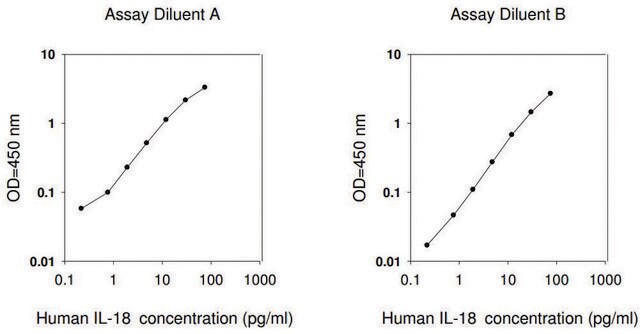
assay range
inter-assay cv: <12%
intra-assay cv: <10%
sensitivity: 2 pg/mL
standard curve range: 12.29-3000 pg/mL
detection method
colorimetric
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... HAVCR1(26762)
General description
Application
Please refer to the attached General ELISA KIT Procedure (sandwich, competitive & Indirect ELISA)
Other Notes
Please type the word sample in the text box provided for lot number.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Met. Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Please refer to KIT Component information
PRTR
Please refer to KIT Component information
FSL
Please refer to KIT Component information
ISHL Indicated Name
Please refer to KIT Component information
ISHL Notified Names
Please refer to KIT Component information
Cartagena Act
Please refer to KIT Component information
JAN Code
キットコンポーネントの情報を参照してください
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service