P8877
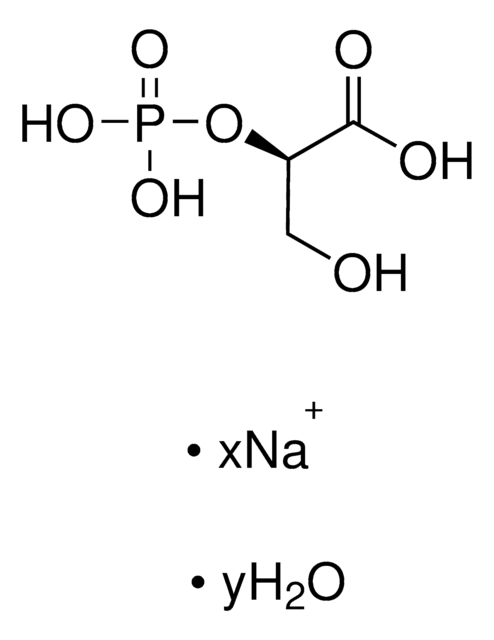
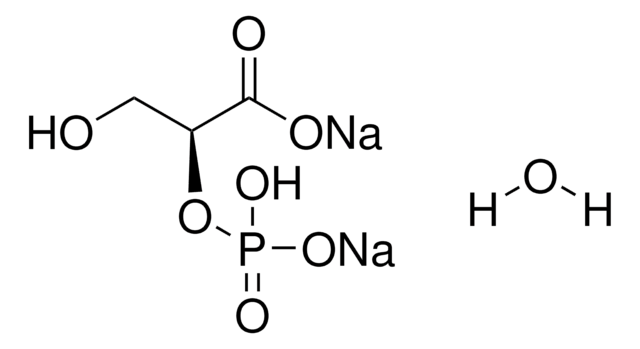
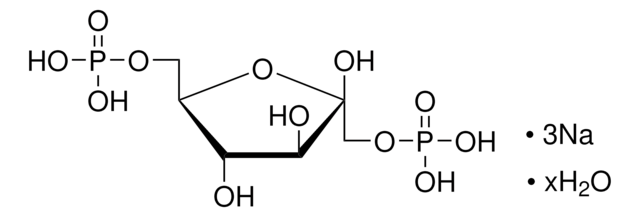
D-(−)-3-Phosphoglyceric acid disodium salt
≥93% dry basis (enzymatic), powder
Synonym(s):
(−)-Disodium D-3-phosphoglycerate, D-Glycerate 3-phosphate disodium salt
About This Item
Recommended Products
biological source
synthetic (inorganic)
Quality Level
Assay
≥93% dry basis (enzymatic)
form
powder
greener alternative product characteristics
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
<14% water (NMR)
color
white
solubility
H2O: 50 mg/mL, clear, colorless to faintly yellow
cation traces
Na: 18-22% (dry basis)
greener alternative category
storage temp.
−20°C
SMILES string
[Na+].[Na+].O[C@H](COP(O)([O-])=O)C([O-])=O
InChI
1S/C3H7O7P.2Na/c4-2(3(5)6)1-10-11(7,8)9;;/h2,4H,1H2,(H,5,6)(H2,7,8,9);;/q;2*+1/p-2/t2-;;/m1../s1
InChI key
RGCJWQYUZHTJBE-YBBRRFGFSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 2 - STOT SE 3
Target Organs
Eyes,Central nervous system, Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
P8877-10G:
P8877-1G:4548174009000
P8877-10MG-KC:
P8877-PM:
P8877-VAR:
P8877-10MG:4548174008997
P8877-5G:4548174009017
P8877-BULK:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about monosaccharide biosynthesis and the metabolism of monosaccharides. A unit of a carbohydrate and the simplest form of a sugar, a monosaccharide cannot be hydrolyzed into a simpler compound.
Review the 10 steps of glycolysis in the Embden-Meyerhof-Parnas glycolytic pathway. Easily compare reaction stages and buy the enzymes for your life science research.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service