About This Item
Recommended Products
biological source
animal (Apis mellifera)
Quality Level
Assay
≥85% (HPLC)
form
lyophilized powder
mol wt
~_2.8 kDa
solubility
water: 5.00-5.20 mg/mL, clear, colorless to faintly yellow
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
foreign activity
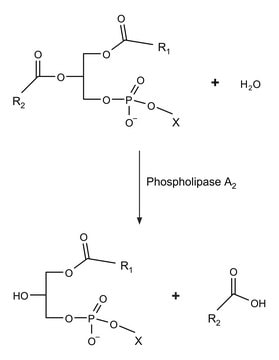
Phospholipase A2 ≤0.5%
Mode of action
cell membrane | interferes
storage temp.
−20°C
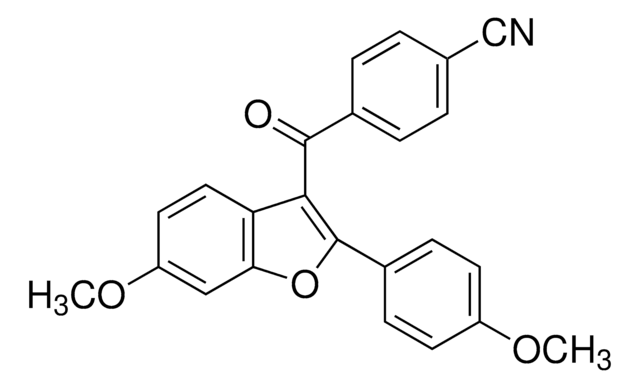
SMILES string
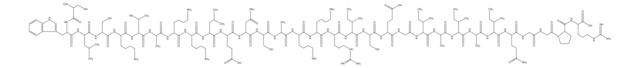
CC[C@H](C)[C@H](NC(=O)CN)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O
InChI
1S/C131H229N39O31/c1-23-71(16)102(163-97(176)60-135)122(194)146-62-98(177)148-74(19)109(181)164-100(69(12)13)124(196)160-88(55-65(4)5)116(188)155-84(41-30-33-51-134)115(187)165-101(70(14)15)125(197)161-90(57-67(8)9)118(190)168-106(77(22)173)128(200)169-105(76(21)172)123(195)147-63-99(178)150-92(58-68(10)11)129(201)170-54-36-44-94(170)121(193)149-75(20)108(180)158-89(56-66(6)7)117(189)166-104(73(18)25-3)127(199)162-93(64-171)120(192)159-91(59-78-61-145-80-38-27-26-37-79(78)80)119(191)167-103(72(17)24-2)126(198)157-83(40-29-32-50-133)111(183)154-85(42-34-52-143-130(139)140)112(184)152-82(39-28-31-49-132)110(182)153-86(43-35-53-144-131(141)142)113(185)156-87(46-48-96(137)175)114(186)151-81(107(138)179)45-47-95(136)174/h26-27,37-38,61,65-77,81-94,100-106,145,171-173H,23-25,28-36,39-60,62-64,132-135H2,1-22H3,(H2,136,174)(H2,137,175)(H2,138,179)(H,146,194)(H,147,195)(H,148,177)(H,149,193)(H,150,178)(H,151,186)(H,152,184)(H,153,182)(H,154,183)(H,155,188)(H,156,185)(H,157,198)(H,158,180)(H,159,192)(H,160,196)(H,161,197)(H,162,199)(H,163,176)(H,164,181)(H,165,187)(H,166,189)(H,167,191)(H,168,190)(H,169,200)(H4,139,140,143)(H4,141,142,144)/t71-,72-,73-,74-,75-,76+,77+,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,100-,101-,102-,103-,104-,105-,106-/m0/s1
InChI key
VDXZNPDIRNWWCW-JFTDCZMZSA-N
Looking for similar products? Visit Product Comparison Guide
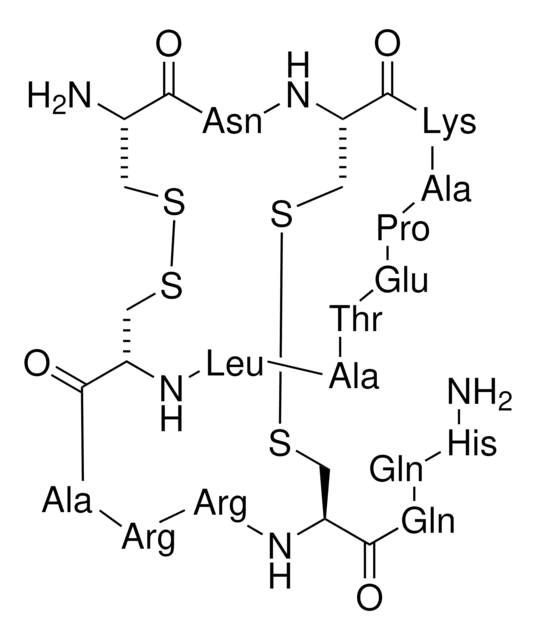
Amino Acid Sequence
General description
Application
- In 3-(4, 5-dimethyl thiazol-2-yl)-2,5diphenyl tetrazolium bromide (MTT) assay to determine its cytotoxicity effect on the growth of human cell lines.
- To study the anti-microbial activity of melittin on the growth of Borrelia burgdorferi in in vitro conditions.
- As a positive control in hemolysis assay and as a cytotoxic agent against HeLa cells.
Biochem/physiol Actions
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
M2272-25MG-PW:
M2272-BULK:
M2272-5MG-PW:
M2272-VAR:
M2272-25MG:
M2272-1MG-PW:
M2272-1MG:
M2272-5MG:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bee Venom (Apis cerana indica) to several human cell lines: HeLa,
WiDr and Vero.
Articles
With bacterial resistance and emerging infectious diseases becoming potential threats to humans, ribosomally synthesized antimicrobial peptides have become a promising focus area in antibiotic research.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service