H4914
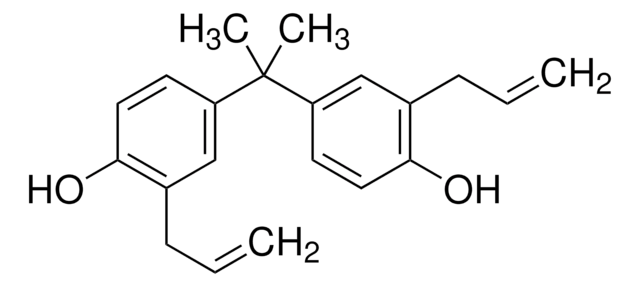
Honokiol
≥98% (HPLC), powder
Synonym(s):
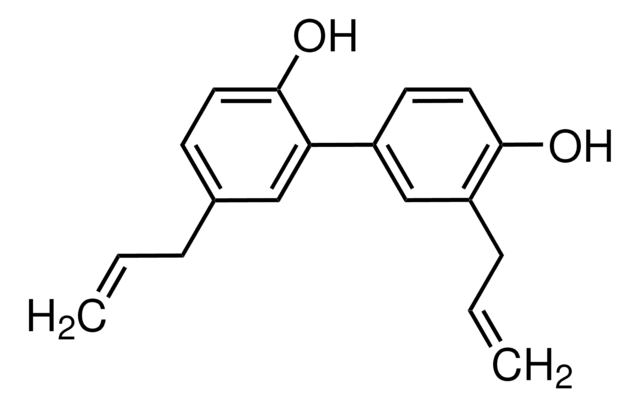
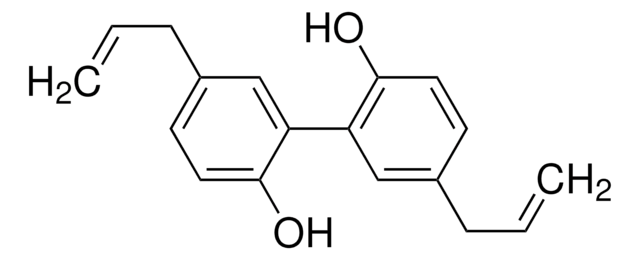
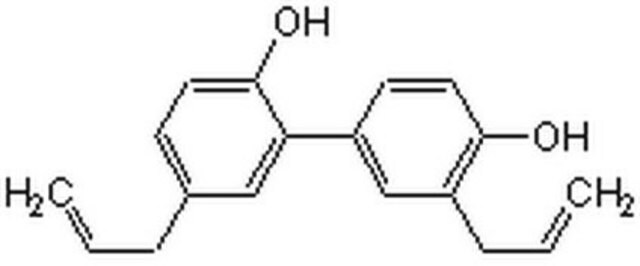
5,3′-Diallyl-2,4′-dihydroxybiphenyl, NSC 293100
About This Item
Recommended Products
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
solubility
DMSO: 36 mg/mL
antibiotic activity spectrum
Gram-positive bacteria
neoplastics
Mode of action
DNA synthesis | interferes
storage temp.
2-8°C
SMILES string
Oc1ccc(cc1CC=C)-c2cc(CC=C)ccc2O
InChI
1S/C18H18O2/c1-3-5-13-7-9-18(20)16(11-13)14-8-10-17(19)15(12-14)6-4-2/h3-4,7-12,19-20H,1-2,5-6H2
InChI key
FVYXIJYOAGAUQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effects on plasmid hSirt3102-399 deacetylation activity
- as an antioxidant to study its cytoprotective role in human ovarian cancer cells (SKOV-3) and Chinese hamster ovary cells (CHOK1)
- to explore its effects on oxidative stress and mitochondrial dysfunction via a sirt3-dependent manner
- for intracerebroventricular (ICV) cannulation
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
H4914-BULK:
H4914-25MG:
H4914-VAR:
H4914-10MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
NF-κB and Inflammation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service