G8415
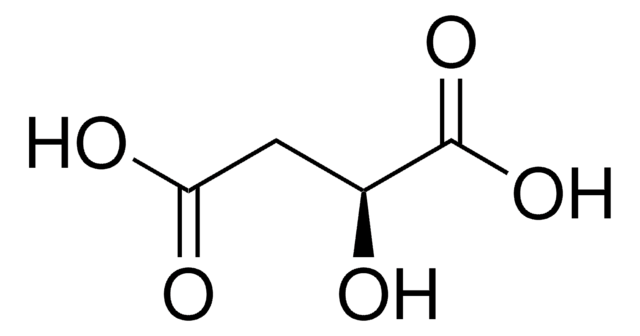
L-Glutamic acid
98.5-100.5%, suitable for cell culture, non-animal source, meets EP testing specifications
Synonym(s):
(S)-2-Aminopentanedioic acid, Glu
About This Item
Recommended Products
Product Name
L-Glutamic acid, from non-animal source, meets EP testing specifications, suitable for cell culture, 98.5-100.5%
biological source
non-animal source
Quality Level
Agency
meets EP testing specifications
Assay
98.5-100.5%
form
powder
technique(s)
cell culture | mammalian: suitable
impurities
endotoxin, tested
color
white
mp
205 °C (dec.) (lit.)
solubility
1 M HCl: 100 mg/mL
density
1.54 g/cm3 at 20 °C
anion traces
chloride (Cl-): ≤200 ppm
sulfate (SO42-): ≤200 ppm
cation traces
As: ≤1 ppm, passes test
Fe: ≤10 ppm, passes test
NH4+: ≤200 ppm, passes test
application(s)
pharmaceutical (small molecule)
SMILES string
N[C@@H](CCC(O)=O)C(O)=O
InChI
1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
InChI key
WHUUTDBJXJRKMK-VKHMYHEASA-N
Gene Information
human ... CCR2(1231) , GRIA1(2890) , GRIA2(2891) , GRIA4(2893) , GRIK1(2897) , GRIK2(2898) , GRIK3(2899) , GRIK5(2901) , GRIN2B(2904) , GRM2(2912) , SLC1A1(6505) , SLC1A2(6506)
rat ... Gria1(50592) , Grik1(29559) , Grik2(54257) , Grik4(24406) , Grin2a(24409) , Grm1(24414) , Grm2(24415) , Grm3(24416) , Grm4(24417) , Grm5(24418) , Grm6(24419) , Grm7(81672) , Slc1a2(29482)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
G8415-1KG:4548173217376
G8415-10MG:4548173907291
G8415-25KG:4548173217390
G8415-VAR:
G8415-10G:4548173217383
G8415-100G:4548173217369
G8415-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
guidance
Articles
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service