G211200
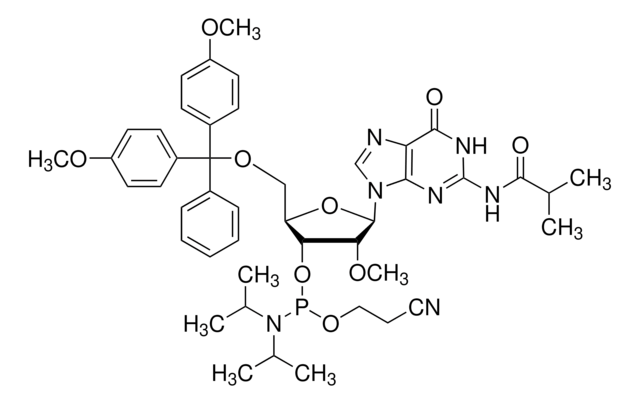
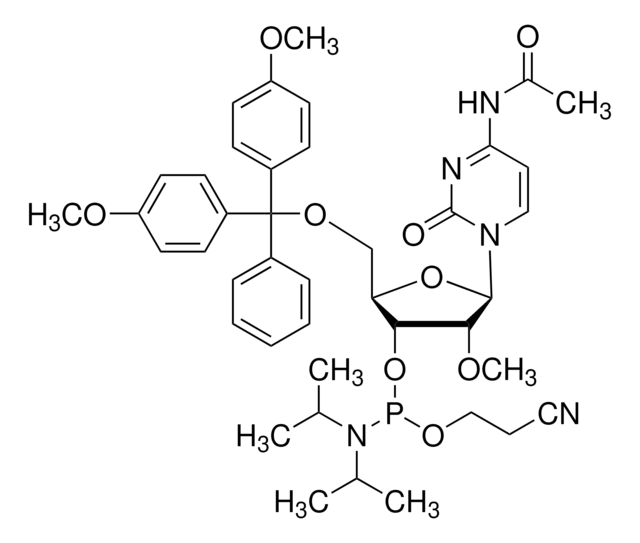
DMT-2′Fluoro-dG(ib) Phosphoramidite
Synonym(s):
DMT-2′Fluoro-dG(ib) Amidite
About This Item
Recommended Products
biological source
non-animal source (no BSE/TSE risk)
product line
Proligo Reagents
Assay
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
form
powder
technique(s)
oligo synthesis: suitable
impurities
≤0.3% water content (Karl Fischer)
≤0.5% P(III) Impurities 100-169ppm (31P-NMR)
≤0.5% single Impurity (reversed phase HPLC)
≤3% residual Solvent content
color
white to off-white
λ
conforms (UV/VIS Identity)
suitability
conforms to structure for H-NMR
conforms to structure for LC-MS
nucleoside profile
base: deoxyguanosine
base protecting group: isobutyryl
2' protecting group: fluoro
5' protecting group: DMT
deprotection: fast/standard
storage temp.
−20°C
SMILES string
COc1ccc(cc1)C(OC[C@H]2O[C@H]([C@H](F)[C@@H]2OP(OCCC#N)N(C(C)C)C(C)C)n3cnc4C(=O)NC(NC(=O)C(C)C)=Nc34)(c5ccccc5)c6ccc(OC)cc6
InChI
1S/C44H53FN7O8P/c1-27(2)40(53)49-43-48-39-37(41(54)50-43)47-26-51(39)42-36(45)38(60-61(58-24-12-23-46)52(28(3)4)29(5)6)35(59-42)25-57-44(30-13-10-9-11-14-30,31-15-19-33(55-7)20-16-31)32-17-21-34(56-8)22-18-32/h9-11,13-22,26-29,35-36,38,42H,12,24-25H2,1-8H3,(H2,48,49,50,53,54)/t35-,36-,38-,42-,61?/m1/s1
InChI key
KJFUMXZZVYMQEU-AOCJBPQJSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Its key features include,
- Can be employed together with DNA or RNA phosphoramidites
- Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA for 10 minutes at 65 °C
- Synthesis of 2′Fluoro oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 3 minutes compared to 90 seconds for DNA monomers)
- Consistent lot-to-lot high purity and performance
- Manufactured under a certified ISO 9001 quality system
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
JAN Code
G211280-2G:4548173334462
G211200-00:
G211200-100G:4548173377315
G211200-500G:
G211200-C:
G211211-01:
G211280-1G:
G211280-0.25G:4548173914374
G211200-AVC:
G211251-01:
G211200-AGL:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service