D2534
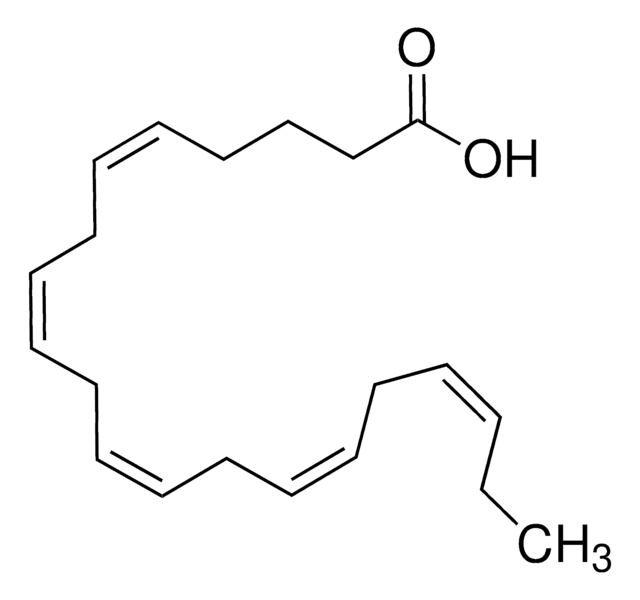
cis-4,7,10,13,16,19-Docosahexaenoic acid
≥98%
Synonym(s):
DHA
About This Item
Recommended Products
biological source
algae
Quality Level
Assay
≥98%
form
liquid
functional group
carboxylic acid
lipid type
omega FAs
shipped in
dry ice
storage temp.
−20°C
SMILES string
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(O)=O
InChI
1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
InChI key
MBMBGCFOFBJSGT-KUBAVDMBSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a component in Dulbecco′s modified Eagle medium (DMEM) for culturing cells to perform docosahexaenoic acid (DHA) treatment
- to study its impact on human induced pluripotent stem cell (iPSC)-derived neuronal at a molecular and cellular level
- in MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to study its cytotoxic effects on three human hepatocellular carcinoma (HCC) cell lines (HepG2, Hep3B, Huh7)
Biochem/physiol Actions
Packaging
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
JAN Code
D2534-1G:
D2534-VAR:
D2534-500MG:
D2534-25MG:
D2534-100MG:
D2534-BULK:
D2534-10MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The potential for the prevention and treatment of cardiovascular disease through increased dietary intake of omega-3 (w-3) fish oils is not a recent scientific discovery.
Lipid Induced Insulin Resistance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service