T176
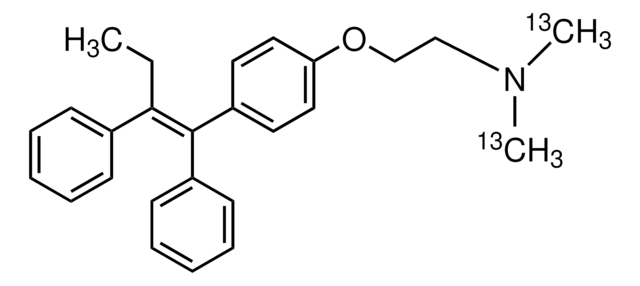
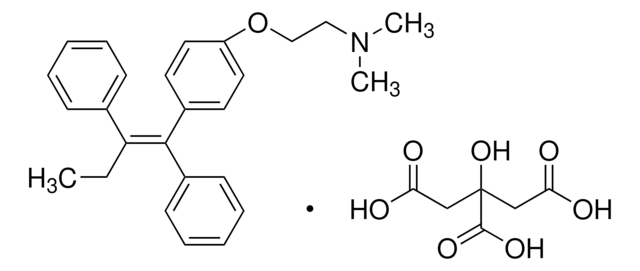
4-Hydroxytamoxifen
analytical standard, (E) and (Z) isomers (50:50)
Synonym(s):
4-(1-[4-(Dimethylaminoethoxy)phenyl]-2-phenyl-1-butenyl)phenol, 4-OHT, cis/trans-4-Hydroxytamoxifen
About This Item
Recommended Products
grade
analytical standard
Quality Level
form
powder
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
solubility
ethanol: 20 mg/mL
antibiotic activity spectrum
neoplastics
application(s)
forensics and toxicology
pharmaceutical (small molecule)
format
neat
Mode of action
enzyme | inhibits
SMILES string
CC\C(c1ccccc1)=C(/c2ccc(O)cc2)c3ccc(OCCN(C)C)cc3.CC\C(c4ccccc4)=C(\c5ccc(O)cc5)c6ccc(OCCN(C)C)cc6
InChI
1S/2C26H29NO2/c2*1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h2*5-17,28H,4,18-19H2,1-3H3/b26-25+;26-25-
InChI key
ZJLDABGSDWXVGE-BDSXMVAQSA-N
Gene Information
human ... ESR1(2099) , ESR2(2100) , ESRRG(2104) , IL6(3569)
rat ... Ar(24208) , Esr1(24890) , Esr2(25149)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Determination of tamoxifen and its three metabolites from dried blood spot (DBS) discs by ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UHPLC-ESI-MS/MS)
- Simultaneous estimation of tamoxifen, 4-hydroxytamoxifen, and endoxifen from DBS samples by UHPLC-MS/MS
- Development and validation of a non-aqueous capillary electrophoretic (NACE) method coupled with capacitively coupled contactless conductivity detection (C4D) to analyze tamoxifen and its three main metabolites after their liquid-liquid extraction (LLE) from human plasma samples obtained from breast cancer patients
- Multi-residue analysis of human plasma samples to quantify tamoxifen and its degradation products using the non-aqueous capillary electrophoretic (NACE) method combined with UV-detection
- Combined detection of tamoxifen and centchroman, along with their degradation products in human plasma samples by LC-ESI-MS/MS
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
T176-BULK:
T176-VAR:
T176-10MG:
T176-50MG:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service