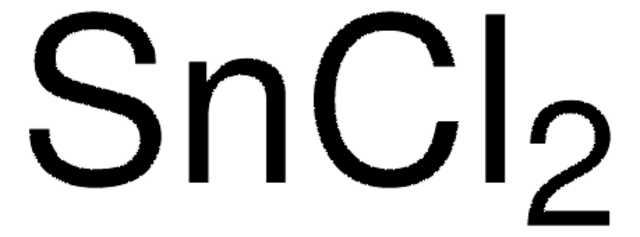204722
Tin(II) chloride
≥99.99% trace metals basis
Synonym(s):
Stannous chloride
About This Item
Recommended Products
vapor pressure
33 hPa (~429 °C)
Quality Level
Assay
≥99.99% trace metals basis
form
crystalline powder
flakes
reaction suitability
reagent type: catalyst
core: tin
pH
2.18 (20 °C)
bp
652 °C (lit.)
mp
246 °C (lit.)
SMILES string
Cl[SnH2]Cl
InChI
1S/2ClH.Sn/h2*1H;/q;;+2/p-2
InChI key
AXZWODMDQAVCJE-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
- aromatic nitro compounds
- nitriles
- cyanosilyl ethers
- organic azides
Application
- To catalyze the addition of diazo sulfones, diazo phosphine oxides and diazo phosphonates to aldehydes to form β-keto sulfones, β-keto phosphine oxides and β-keto phosphonates, respectively.
- Along with trityl chloride, to catalyze the aldol reaction of silyl enol ethers with acetals or aldehydes and the Michael reaction of silyl enol ethers with α,β-unsaturated ketones.
- As a promoter in the allylic amination of allylic alcohols with amines in the presence of palladium catalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 Oral - STOT SE 3
Target Organs
Cardio-vascular system, Respiratory system
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
204722-BULK:
204722-VAR:
204722-10G:4548173114477
204722-50G:4548173114484
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service