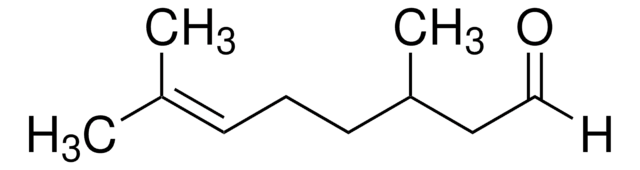
05818
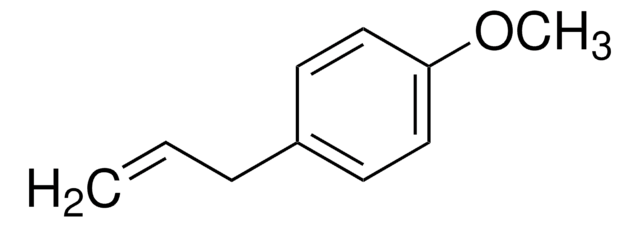
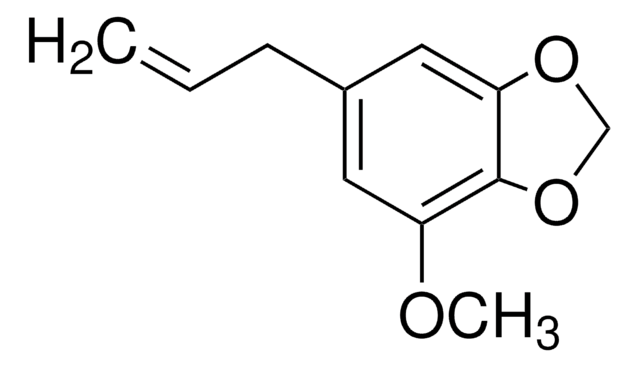
4-Allylanisole
analytical standard
Synonym(s):
p-Allylphenyl methyl ether, p-Methoxyallylbenzene, Chavicol methyl ether, Estragole, Isoanethole, Methyl chavicol
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥98.5% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.521 (lit.)
n20/D 1.521
bp
215-216 °C (lit.)
density
0.965 g/mL at 25 °C (lit.)
application(s)
food and beverages
format
neat
SMILES string
COc1ccc(CC=C)cc1
InChI
1S/C10H12O/c1-3-4-9-5-7-10(11-2)8-6-9/h3,5-8H,1,4H2,2H3
InChI key
ZFMSMUAANRJZFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 3 petroleums
Hazardous rank III
Water insoluble liquid
JAN Code
05818-1ML:
05818-VAR:
05818-BULK:
05818-5ML:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service