SCR527M
Alzheimer′s In A Dish™ PSEN1-RFP Lentivirus
Synonym(s):
PSEN1-RFP, ReNcell 3D Alzheimers Model, ADID 3D Neural Cell Model
About This Item
Recommended Products
species reactivity
human
Quality Level
technique(s)
cell culture | mammalian: suitable
cell culture | stem cell: suitable
cell differentiation: suitable
General description
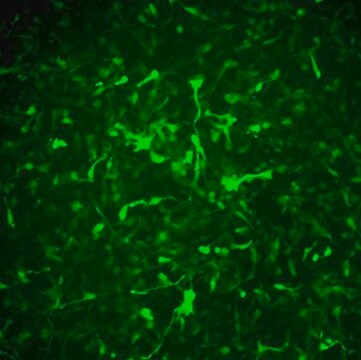
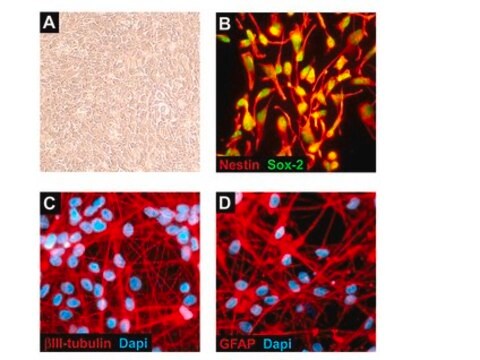
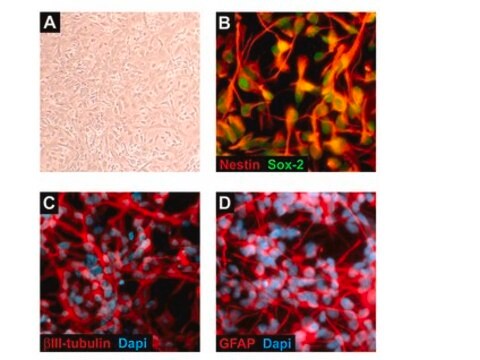
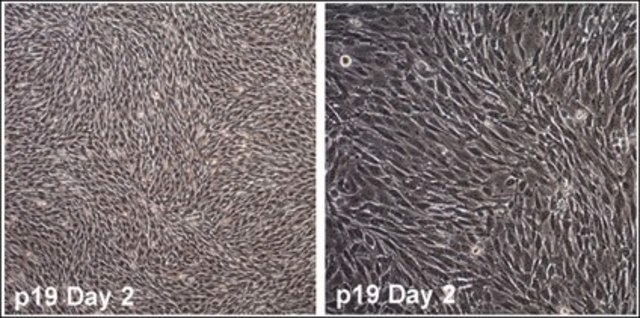
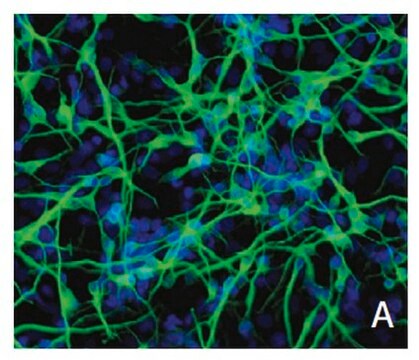
Recently a 3D model using genetically engineered human neural stem cells that overexpress FAD mutations was reported to recapitulate the two pathological hallmarks of AD: b-amyloid plaques and neurofibrillary tangles. Lentiviruses expressing FAD mutations in human APP with both the K670N/M671L (Swedish) and V717I (London) mutations (APPSL) and/or PSEN1 with the E9 mutation (PSEN1(E9)) and APPSL/PSEN1(E9) along with fluorescent proteins as reporters for viral infection (see below), were used to transfect ReNcell VM human neural stem cells. FACS was utilized to enrich for cells with the highest transgene expressions followed by encapsulation of the sorted cells in a 3D Matrigel culture system. After approximately 6 weeks of differentiation, aggregation of AB was observed. Robust accumulation of phosphorylated tau along neurofibrillary tangles was readily detectable after 10-14 weeks.
The Alzheimer’s In A Dish<TMSYMBOL></TMSYMBOL> PSEN1-RFP Lentivirus is a necessary reagent to set up the Alzheimer’s 3D culture system. The lentivirus contains the PSEN1 FAD mutations and can be used to infect human neural cells. The RFP tag enables assessment of viral infectivity and allows FACS sorting of the highest expressing cells.
Application
Stem Cell Research
Neuroscience
Components
Note: For exact viral titer, refer to the label on the vial.
Quality
Storage and Stability
Lentivirus is stable for at least 6 months when stored at -80°C. After first thaw, place immediately on ice and store in working aliquots to avoid further freeze thaws. Avoid freeze thaws as this will result in a decrease in the virus titer.
Important Safety Note:
• Replication-defective lentiviral vectors are not known to cause any diseases in humans or animals. However, lentiviruses can integrate into the host cell genome and thus pose some risk of insertional mutagenesis.
• Wear gloves when using this product. Avoid skin contact or ingestion of all reagents and chemicals used in this protocol.
• Lentivirus is a risk group 2 and should be handled under approved Biosafety Level 2 (BSL2) controls.
Legal Information
Storage Class Code
12 - Non Combustible Liquids
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
SCR527:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
A stem cell culture protocol to generate 3D NSC models of Alzheimer’s disease using ReNcell human neural stem cell lines.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service