General description
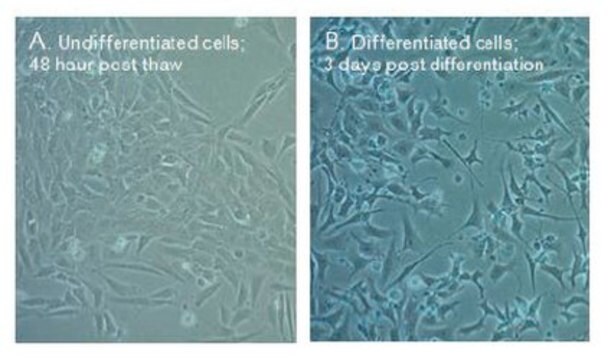
The hybrid MN9D immortalized dopaminergic neuronal cell line was generated by fusion between rostral mesencephalic neurons from a 14-day-old C57BL/6J mouse embryo and N18TG2 neuroblastoma cells, a sympathetic nervous system cancer of A/Jax background.1 MN9D cells produce dopamine (DA) and express tyrosine hydroxylase (TH) as well as aromatic amino acid decarboxylase (AADC).2 MN9D cells readily aggregate with one another or other embryonic brain cells2 and can be transfected using calcium phosphate precipitation3 or Lipofectamine4.Undifferentiated or differentiated MN9D cells are extensively used to model dopaminergic neurons and to test mechanisms and potential therapeutics relevant to the loss of DA neurons in Parkinson′s disease. Differentiated with butyric acid or glial cell line-derived neurotrophic factor (GDNF) followed by butyric acid, MN9D cells only partially recapitulate the electrophysiological properties of midbrain DA neurons. Optimizing MN9D differentiation further using one or a combination of growth or other factors may yield an improved model system for Parkinson′s disease studies in vitro.5
Application
The mechanisms by which central neurons develop axons and establish functional connections with their target cells as well as the role of such connections in cell survival are important developmental neurobiology questions studied using in vitro cell-reaggregation systems, both for central dopaminergic and cholinergic neurons. Hybrid clonal cells facilitate the study of trophic interactions between mesencephalic dopaminergic neurons and their target cells. References1.Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC, Heller A. (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res 552(1):67–76.2.Choi HK, Won L, Roback JD, Wainer BH, Heller A. (1992) Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci USA 89(19):8943–8947.3.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22(8):3090-30994.Cavanaugh JE, Jaumotte JD, Lakoski JM, Zigmond MJ. (2006) Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress. J Neurosci Res 84(6):1367-13755.Rick CE, Ebert A , Virag T, Bohn MC, Surmeier DJ. (2006) Differentiated dopaminergic MN9D cells only partially recapitulate the electrophysiological properties of midbrain dopaminergic neurons. Dev Neurosci 28(6):528-537.
• Each vial contains ≥ 1X10⁶ viable cells.• MN9D cells are verified to be of mouse origin and negative for rat, Chinese hamster, Golden Syrian hamster, human, and non-human primate interspecies contamination, as assessed by a Contamination Clear panel by Charles River Animal Diagnostic Services.• Cells tested negative for infectious diseases by a Mouse Essential CLEAR panel by Charles River Animal Diagnostic Services.• Cells tested negative for mycoplasma.
Other Notes
This product is intended for sale and sold solely to academic institutions for internal academic research use per the terms of the “Academic Use Agreement” as detailed in the product documentation. For information regarding any other use, please contact licensing@emdmillipore.com.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.