8.55060
Rink Acid resin (100-200 mesh)
Novabiochem®
Synonym(s):
4-(2′,4′-Dimethoxyphenyl-hydroxymethyl)-phenoxy resin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
13111023
NACRES:
NA.22
Recommended Products
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
alcohol
storage temp.
2-30°C
General description
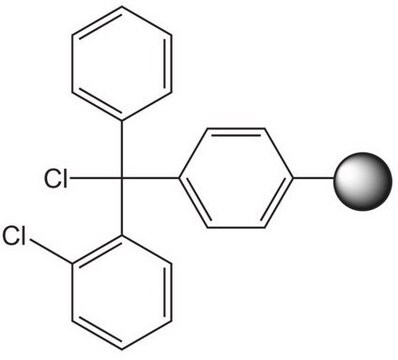
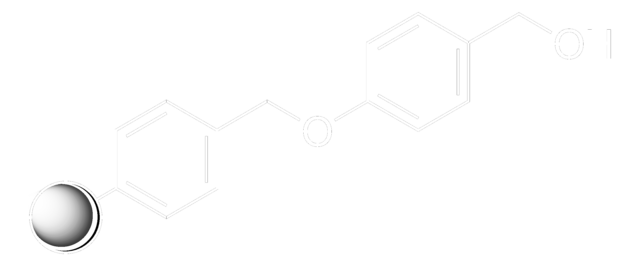
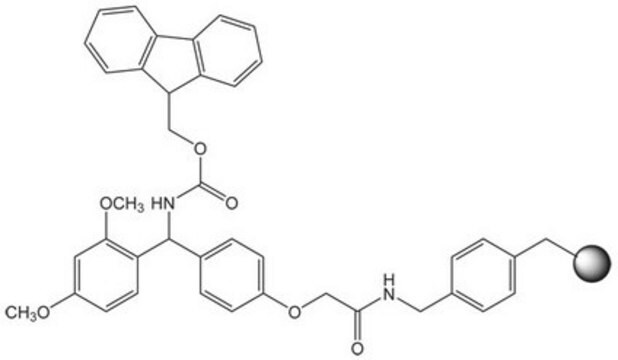
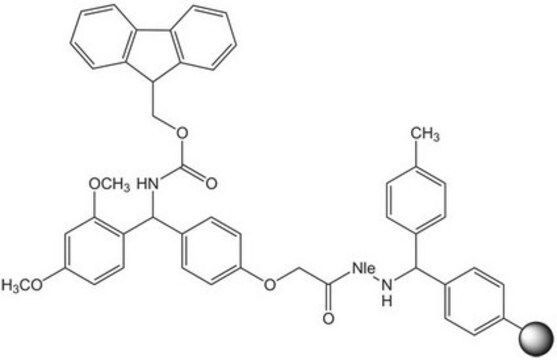
Rink acid resin is a super acid-labile support for the solid phase immobilization of carboxylic acids [1] by Fmoc SPPS. Cleavage can be effected with as little as 10% AcOH in DCM, providing highly acid-sensitive products in high yields and purities. However, care must be taken to prevent product loss during synthetic manipulations [2], owing to the extreme acid sensitivity of this support. Treatment with HCl in THF [3] or Ph2PCl2 [4] has been shown to efficiently convert this resin to the corresponding benzhydryl chloride, to which can be coupled a wide range of functional groups: hydroxylamines [3], alcohols, amines, acids and thiols [4]. Rink acid resin has also been converted to a trifluoroacetate with TFA and used in a similar manner to immobilize amines, thiols, alcohols, and phenols [5]. In a more detailed study, the same authors found 1 M trifluoroacetic anhydride in 2,6-lutidine to give superior results with less degradation of the linker [6, 7]. Rink resin trifluoroacetate has also been used to prepare purines [8, 9].Cleavage of amines and alcohols from this support has been carried out with either 5% TFA in DCM [4] or 20% TFA in DCM [5]; thiols were released with either 5% TFA in DCM [4] or 95% aq. TFA [5].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] H. Rink (1987) Tetrahedron Lett., 28, 3787.
[2] H. U. Immer, et al. in ′Peptides, Chemistry, Structure &Biology, Proc. 11th American Peptide Symposium′, J. E. Rivier & G. R. Marshall (Eds), ESCOM, Leiden, 1990, pp. 1054.
[3] S. L. Mellor & W. C. Chan (1997) J. Chem. Soc., Chem. Commun., 2005.
[4] R. S. Garigipati (1997) Tetrahedron Lett., 38, 6807.
[5] W.K.-D. Brill in ′First Conference on Synthetic Organic Chemistry′, www.mdpi.org/ecsoc, 1997.
[6] W.K.-D. Brill (1998) Syn. Lett., 906.
[7] R. A. Tommesi, et al. (1998) Tetrahedron Lett., 39, 5477.
[8] W.K.-D. Brill (2001) Syn. Lett., 1097.
[9] W.K.-D. Brill & C. Riva-Toniolo (2001) Tetrahedron Lett., 42, 65`5.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] H. Rink (1987) Tetrahedron Lett., 28, 3787.
[2] H. U. Immer, et al. in ′Peptides, Chemistry, Structure &Biology, Proc. 11th American Peptide Symposium′, J. E. Rivier & G. R. Marshall (Eds), ESCOM, Leiden, 1990, pp. 1054.
[3] S. L. Mellor & W. C. Chan (1997) J. Chem. Soc., Chem. Commun., 2005.
[4] R. S. Garigipati (1997) Tetrahedron Lett., 38, 6807.
[5] W.K.-D. Brill in ′First Conference on Synthetic Organic Chemistry′, www.mdpi.org/ecsoc, 1997.
[6] W.K.-D. Brill (1998) Syn. Lett., 906.
[7] R. A. Tommesi, et al. (1998) Tetrahedron Lett., 39, 5477.
[8] W.K.-D. Brill (2001) Syn. Lett., 1097.
[9] W.K.-D. Brill & C. Riva-Toniolo (2001) Tetrahedron Lett., 42, 65`5.
Linkage
Replaces: 01-64-0012
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substition of the Fmoc-NH2 loaded resin): 0.35 - 0.80 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
Appearance of substance (visual): beads
Loading (determined from the substition of the Fmoc-NH2 loaded resin): 0.35 - 0.80 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service