219373
Cathepsin G, Human Neutrophil
Cathepsin G, Human Neutrophil, CAS 107200-92-0, is a purified native cathepsin G. Acts as a potent agonist of human platelet activation leading to their aggregation.
Synonym(s):
Cathepsin G, Human Neutrophil
About This Item
Recommended Products
biological source
human neutrophils
Quality Level
description
Merck USA index - 14, 1905
Assay
≥95% (SDS-PAGE)
form
lyophilized solid (Salt-free)
specific activity
≥2 units/mg protein
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
technique(s)
inhibition assay: suitable
suitability
suitable for molecular biology
application(s)
life science and biopharma
shipped in
ambient
storage temp.
−20°C
Gene Information
human ... CTSG(1511)
General description
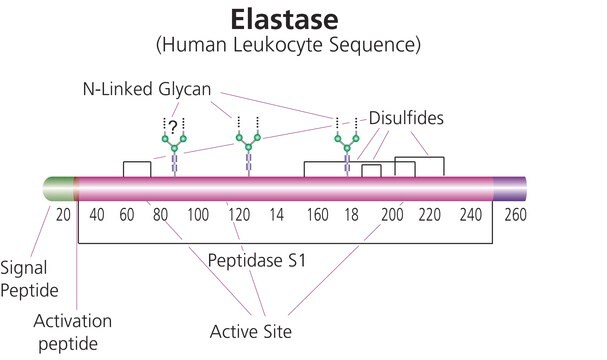
Cathepsin G is stored in its active form in azurophil granules of neutrophils or monocytes. Its mature form contains one potential glycan-binding site and three disulfide bonds.
Application
Biochem/physiol Actions
Warning
Unit Definition
Preparation Note
Reconstitute in 150 mM NaCl, 50 mM sodium acetate buffer, pH 5.5.
Reconstitution
Other Notes
Shamamian, P., et al. 2001. J. Cell Physiol.189, 197.
Groutas, W.C., et al. 1993. Biochem. Biophys. Res. Commun.197, 730.
Stone, P.J., et al. 1993. Biochem. Biophys. Res. Commun.197, 130.
Groutas, W.C., et al. 1992. Arch. Biochem. Biophys.294, 144.
Maison, C.M., et al. 1991. J. Immunol.147, 921.
Travis, J. 1988. Am. J. Med.84, 37.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
219373-100MIU:
219373-0U:
219373-500MIU:
219373-U:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service