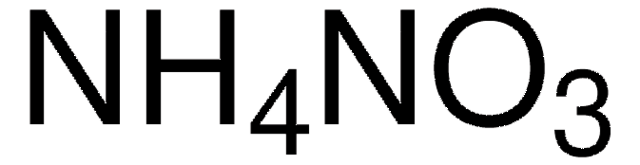1.01188
Ammonium nitrate
for analysis EMSURE® ACS
Synonym(s):
Ammonium nitrate, Nitric acid ammonia, Ammonia nitrate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
product line
EMSURE®
Assay
≥95.0% (alkalimetric)
form
solid
potency
2462 mg/kg LD50, oral (Rat)
impurities
≤0.005% Insoluble matter
ign. residue
≤0.01% (as sulfate)
total organic carbon (TOC) residue (Combustable matter, including organic components)
≤0.2% (as carbon)
pH
4.5-7.0 (20 °C, 100 g/L in H2O)
bp
210 °C/15.0 hPa
mp
169 °C
solubility
1920 g/L
density
1.72 g/cm3 at 20 °C
bulk density
600‑700 kg/m3
anion traces
chloride (Cl-): ≤0.0003%
nitrite (NO2-): ≤0.0005%passes test
phosphate (PO43-): ≤0.0005%
sulfate (SO42-): ≤0.002%
cation traces
Ca: ≤0.003%
Fe: ≤0.0002%
heavy metals (as Pb): ≤0.0005%
storage temp.
2-30°C
InChI
1S/NO3.H3N/c2-1(3)4;/h;1H3/q-1;/p+1
InChI key
DVARTQFDIMZBAA-UHFFFAOYSA-O
Application
- Role of gas-particle conversion of ammonia in haze pollution under ammonia-rich environment in Northern China and prospects of effective emission reduction.: This study investigates the role of ammonium nitrate in haze pollution, emphasizing its environmental impact and the potential for emission reduction strategies (Zou et al., 2024).
- Electrocatalytic Nitrate Reduction on Metallic CoNi-Terminated Catalyst with Industrial-Level Current Density in Neutral Medium.: This paper explores the use of ammonium nitrate in electrocatalytic processes for nitrate reduction, demonstrating its application in industrial catalysis (Wei et al., 2024).
- Thermal annealing gradient tailoring on ammonium nitrate-capped CdS nanospheres synthesized via green precipitation.: The study focuses on the synthesis of CdS nanospheres using ammonium nitrate, emphasizing its applications in nanomaterial engineering (Kok Sheng & Alrababah, 2024).
Linkage
Analysis Note
Insoluble matter: ≤ 0.005 %
pH-value (5 %; water, 25 °C): 4.5 - 6.0
Chloride (Cl): ≤ 0.0003 %
Nitrite (NO₂):
≤ 0.0005 %
: passes test
Phosphate (PO₄): ≤ 0.0005 %
Sulfate (SO₄): ≤ 0.002 %
Heavy metals (as Pb): ≤ 0.0005 %
Ca (Calcium): ≤ 0.003 %
Fe (Iron): ≤ 0.0002 %
Residue on ignition (as sulfate): ≤ 0.01 %
The following parameter from a double TOC determination of one batch:
Combustible matter, including organic components (as carbon) ≤ 0.2 %
(meets the specified value)
In accordance with class 5.1 UN 1942.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3
Storage Class Code
5.1C - Ammonium nitrate and ammonium nitrate containing preparations
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Oxidizing solids
Nitrates
Hazardous rank III
3rd oxidizing solid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service