W404926
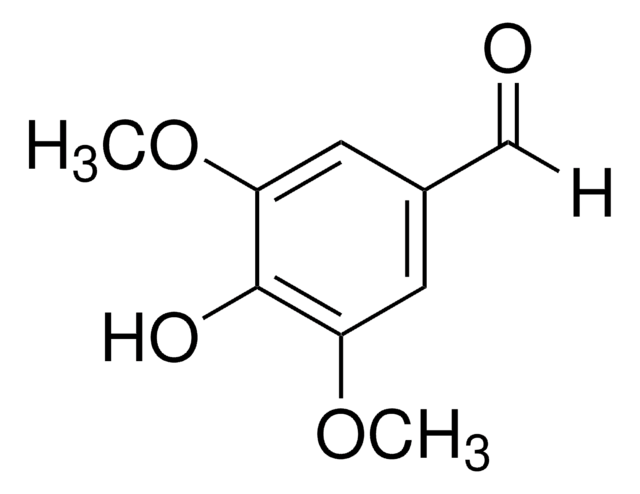
Syringaldehyde
≥98%, FG
Synonym(s):
3,5-Dimethoxy-4-hydroxybenzaldehyde, 4-Hydroxy-3,5-dimethoxybenzaldehyde
About This Item
Recommended Products
biological source
synthetic
grade
FG
reg. compliance
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 110
Assay
≥98%
bp
192-193 °C/14 mmHg (lit.)
mp
110-113 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
green; sweet
SMILES string
COc1cc(C=O)cc(OC)c1O
InChI
1S/C9H10O4/c1-12-7-3-6(5-10)4-8(13-2)9(7)11/h3-5,11H,1-2H3
InChI key
KCDXJAYRVLXPFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Dual action of benzaldehydes: Inhibiting quorum sensing and enhancing antibiotic efficacy for controlling Pseudomonas aeruginosa biofilms.: This study investigates the dual action of syringaldehyde and other benzaldehydes in inhibiting quorum sensing and enhancing the efficacy of antibiotics against Pseudomonas aeruginosa biofilms, offering potential applications in antimicrobial therapies (Leitão et al., 2024).
- Development of Syringaldehyde as an Agonist of the GLP-1 Receptor to Alleviate Diabetic Disorders in Animal Models.: Research highlights the development of syringaldehyde as a novel agonist of the GLP-1 receptor, demonstrating significant potential in alleviating diabetic disorders in animal models (Lee et al., 2024).
- Aqueous-Phase Photoreactions of Mixed Aromatic Carbonyl Photosensitizers Yield More Oxygenated, Oxidized, and less Light-Absorbing Secondary Organic Aerosol (SOA) than Single Systems.: The study reveals that syringaldehyde, as part of mixed aromatic carbonyl photosensitizers, leads to the formation of highly oxygenated and oxidized secondary organic aerosols, impacting atmospheric chemistry and air quality (Mabato et al., 2024).
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
W404926-100G:4548173343747
W404926-VAR:
W404926-BULK:
W404926-1KG:4548173343754
W404926-5KG:4548173343761
W404926-SAMPLE:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service