W342807
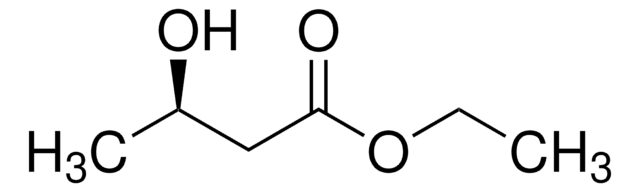
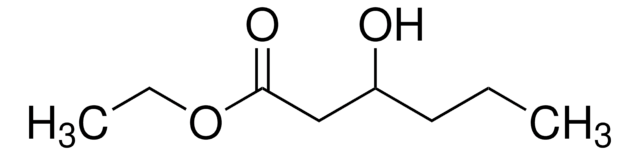
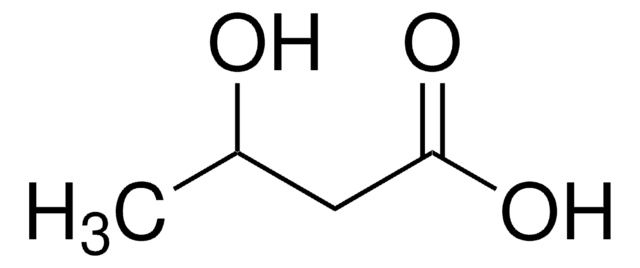
Ethyl 3-hydroxybutyrate
≥97%, FG
Synonym(s):
grape butyrate
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Assay
≥97%
refractive index
n20/D 1.42 (lit.)
bp
170 °C (lit.)
density
1.017 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
grape; green; fruity
SMILES string
CCOC(=O)CC(C)O
InChI
1S/C6H12O3/c1-3-9-6(8)4-5(2)7/h5,7H,3-4H2,1-2H3
InChI key
OMSUIQOIVADKIM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Deciphering the immobilization of lipases on hydrophobic wrinkled silica nanoparticles.: This study explores the immobilization of lipases on hydrophobic wrinkled silica nanoparticles, highlighting the potential of these nanoparticles in biocatalysis applications, which can be critical for biochemical synthesis processes involving compounds like Ethyl 3-hydroxybutyrate. (Pota et al., 2024).
- Exploring the Therapeutic Potential of Ethyl 3-Hydroxybutyrate in Alleviating Skeletal Muscle Wasting in Cancer Cachexia.: This research investigates the therapeutic effects of Ethyl 3-hydroxybutyrate in reducing muscle wasting in cancer patients, demonstrating its potential biochemical applications in medical treatments. (Zhou et al., 2023).
- High-Pressure Depolymerization of Poly(lactic acid) (PLA) and Poly(3-hydroxybutyrate) (PHB) Using Bio-Based Solvents: A Way to Produce Alkyl Esters Which Can Be Modified to Polymerizable Monomers.: This study explores the depolymerization of biopolymers using bio-based solvents, resulting in alkyl esters like Ethyl 3-hydroxybutyrate, highlighting its applications in sustainable biochemical processes. (Jašek et al., 2022).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
170.6 °F - closed cup
Flash Point(C)
77 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Flammable liquids
Type 3 petroleums
Hazardous rank III
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
W342807-100G-K:4.548173977836E12
W342807-1KG-K:4.54817397785E12
W342807-10KG-K:4.548173977843E12
W342807-25KG-K:4.548173349091E12
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service