W248924
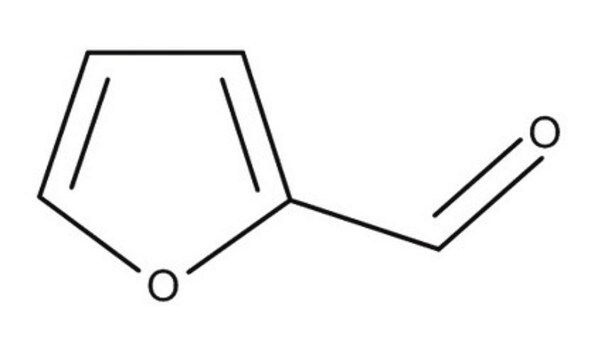
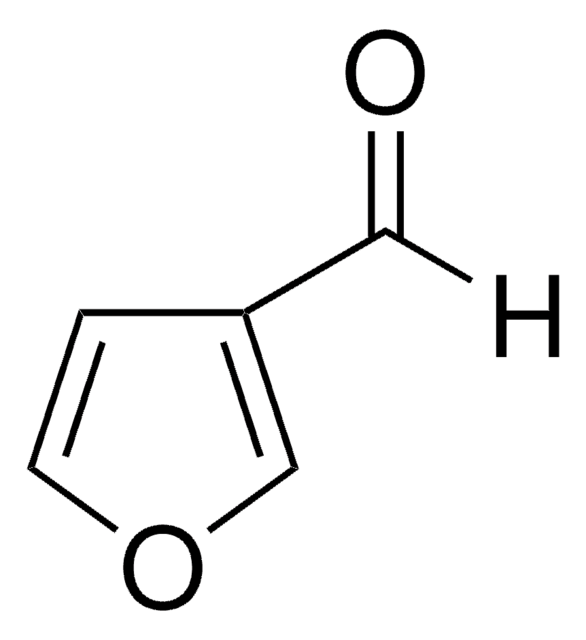
Furfural
natural, ≥98%, FCC, FG
Synonym(s):
2-Furaldehyde, Furan-2-carboxaldehyde
About This Item
Recommended Products
grade
FG
Fragrance grade
Halal
Kosher
natural
Quality Level
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 175.105
vapor density
3.31 (vs air)
vapor pressure
13.5 mmHg ( 55 °C)
Assay
≥98%
form
liquid
autoignition temp.
599 °F
expl. lim.
19.3 %
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.525 (lit.)
bp
162 °C (lit.)
mp
−36 °C (lit.)
solubility
95% ethanol: soluble 1 mL/mL, clear
density
1.16 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
greener alternative category
, Aligned
Organoleptic
almond; baked; bread; woody; sweet
SMILES string
[H]C(=O)c1ccco1
InChI
1S/C5H4O2/c6-4-5-2-1-3-7-5/h1-4H
InChI key
HYBBIBNJHNGZAN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 4: Flammable liquids
Type 2 petroleums
Hazardous rank III
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
W248924-1KG-K:4548173969961
W248924-10KG:
W248924-5KG:
W248924-SAMPLE-K:
W248924-1KG:
W248924-SAMPLE:
W248924-10KG-K:4548173969954
W248924-BULK-K:
W248924-5KG-K:4548173969978
W248924-VAR-K:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service