G4802
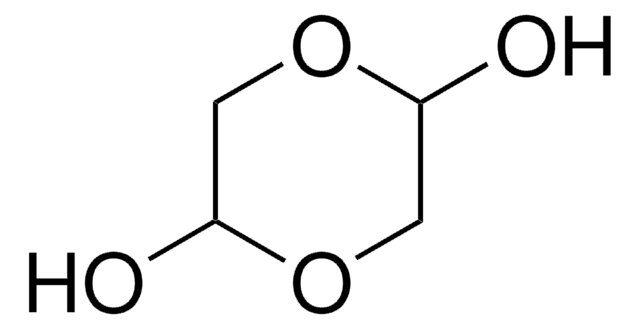
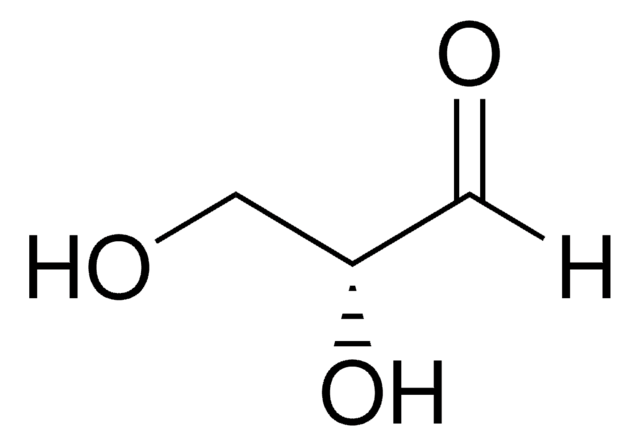
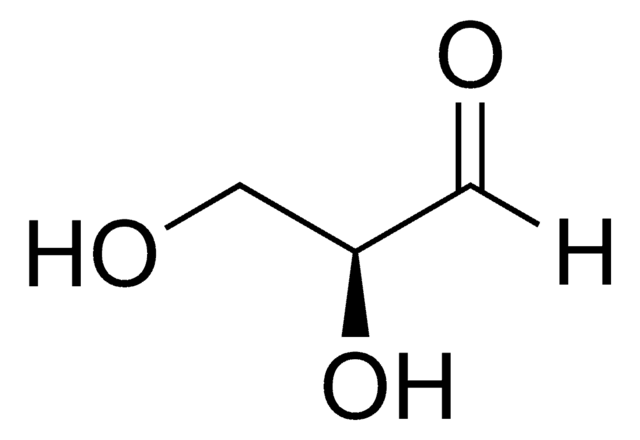
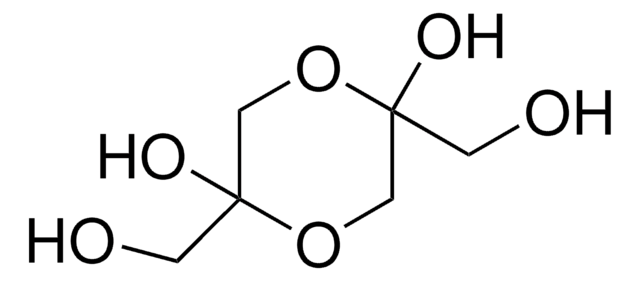
DL-Glyceraldehyde, dimer
95%
Synonym(s):
3,6-Dihydroxy-1,4-dioxane-2,5-dimethanol
About This Item
Recommended Products
Quality Level
Assay
95%
mp
144-145 °C (lit.)
storage temp.
2-8°C
SMILES string
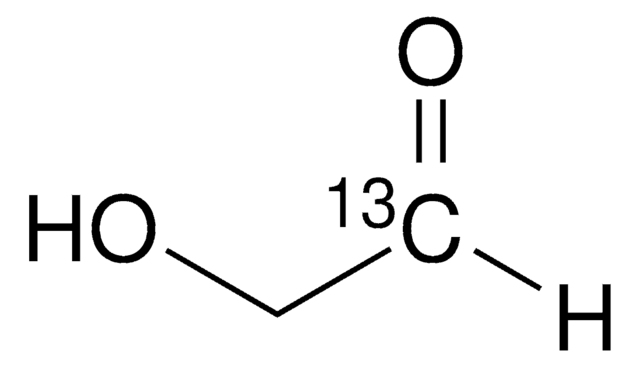
OC[C@H]1O[C@@H](O)[C@H](CO)O[C@@H]1O
InChI
1S/2C3H6O3/c2*4-1-3(6)2-5/h2*1,3,5-6H,2H2
InChI key
NGNVWCSFFIVLAR-UHFFFAOYSA-N
General description
Application
- Aldehyde Reductase Activity: The enzymatic properties of DL-Glyceraldehyde dimer were examined in the context of aldehyde reductases from Euonymus japonica leaves, providing insights into the reduction processes of aldose sugars, which are significant for understanding stress responses in plants (Negm, 1986).
- Enzymatic Reductase Functions: DL-Glyceraldehyde dimer was studied in the purification and characterization of human liver aldehyde reductases, focusing on its role in metabolic detoxification, crucial for pharmaceutical applications involving drug metabolism and toxicity (Petrash and Srivastava, 1982).
- Protozoan Metabolic Pathways: The enzymatic characterization of aldehyde reductase from Crithidia fasciculata, with DL-Glyceraldehyde dimer as a substrate, provided insights into the metabolic pathways of protozoans, important for developing treatments against parasitic infections (Kobayashi, 1982).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
G4802-5G:
G4802-VAR:
G4802-1G:
G4802-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service