D34108
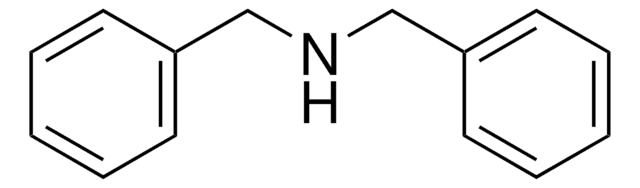
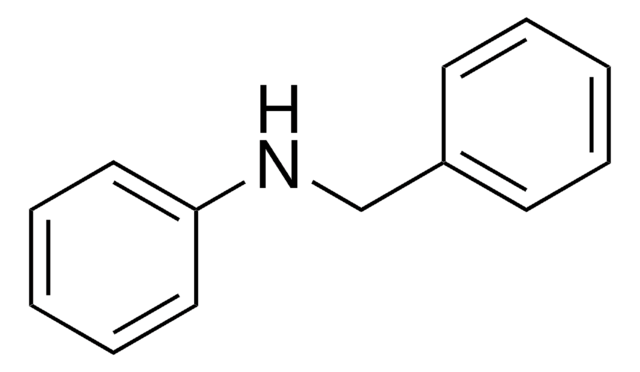
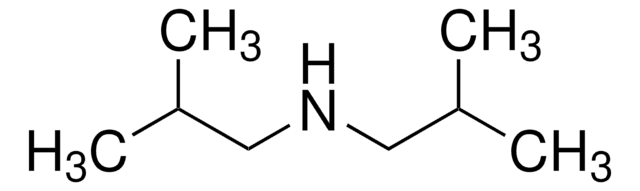
Dibenzylamine
97%
Synonym(s):
(N-Benzylaminomethyl)benzene, Bibenzylamine, DBA, Dibenzylamine (8CI), N,N-Dibenzylamine, N-(Phenylmethyl)benzenemethanamine, N-Benzyl-1-phenylmethanamine, N-Benzylbenzylamine
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.574 (lit.)
bp
300 °C (lit.)
mp
−26 °C (lit.)
density
1.026 g/mL at 25 °C (lit.)
SMILES string
C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H15N/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2
InChI key
BWLUMTFWVZZZND-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Environmental monitoring in shale gas wastewater: Dibenzylamine was identified among the hazardous substances in shale gas wastewater, with research characterizing its concentration and distribution in the Upper Yangtze River, contributing to improved environmental management practices (Tang et al., 2024).
- Advancements in organic synthesis: Dibenzylamine was used in a novel synthetic strategy for (L)-Monomethyl Tyrosine via bulky ′forced-traceless′ regioselective Pd-catalyzed C(sp(2))-H activation, showcasing its utility in pharmaceutical compound development (Illuminati et al., 2023).
- Application in crystallography: The crystal structure of di-benzyl-ammonium was elucidated, providing insights into molecular interactions and potential applications in material science and drug design (Traoré et al., 2023).
- Utilization in green chemistry: Dibenzylamine facilitated a green approach towards Triazole forming reactions, aiming to develop anticancer drugs by minimizing environmental impact and enhancing reaction efficiency (Rastogi et al., 2023).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 3 petroleums
Hazardous rank III
Water insoluble liquid
JAN Code
D34108-100G:
D34108-500G:
D34108-5G:
D34108-VAR:
D34108-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service