D198501
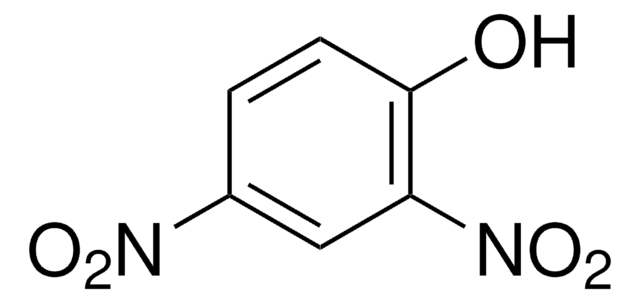
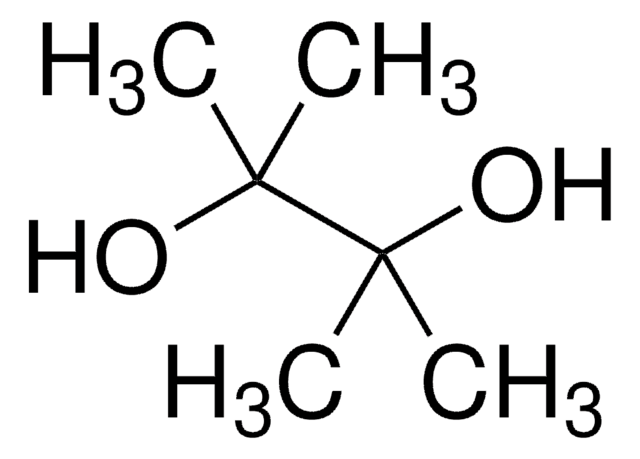
2,4-Dinitrophenol
moistened with water, ≥98.0%
Synonym(s):
α-Dinitrophenol, 2,4-DNP, DNP
About This Item
Recommended Products
vapor density
6.35 (vs air)
Quality Level
Assay
≥98.0%
contains
≥15% water
mp
108-112 °C (lit.)
SMILES string
Oc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C6H4N2O5/c9-6-2-1-4(7(10)11)3-5(6)8(12)13/h1-3,9H
InChI key
UFBJCMHMOXMLKC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a reactant for catalytic reduction reactions.
- To activate carboxylic acids by converting them into dinitrophenyl (DNP) esters.
- To prepare the corresponding ester via acylation reaction using isobutyric anhydride catalyzed by hafnium triflate.
- As an effective cocatalyst to accelerate the activity and enantioselectivity of primary amine organocatalyst derived from natural primary amino acids for direct asymmetric aldol reaction.
- As an alternative activator to tetrazoles in the reaction of phosphoroamidites with nucleosides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Desen. Expl. 4 - STOT RE 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Poisonous substance
PRTR
Class I Designated Chemical Substances
FSL
Group 5: Self-reactive substances
Nitro compounds
Hazardous rank II
2nd self-reactive materials
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
D198501-VAR:
D198501-100G:4548173193779
D198501-5G:4548173193793
D198501-1KG:4548173193786
D198501-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidative stress is mediated, in part, by reactive oxygen species produced by multiple cellular processes and controlled by cellular antioxidant mechanisms such as enzymatic scavengers or antioxidant modulators. Free radicals, such as reactive oxygen species, cause cellular damage via cellular.
Separation of 2-Chlorophenol; 2,4-Dichlorophenol; 2,4,6-Tribromophenol; 2,4,6-Trichlorophenol; 2,4-Dinitrophenol; Pentafluorophenol; 2-Methylphenol, analytical standard; 2,3,4,6-Tetrachlorophenol; Pentachlorophenol; 4-Nitrophenol; 2-Bromophenol; 2,3,5,6-Tetrachlorophenol; 2,3,5-Trichlorophenol; 4-Chloro-3-methylphenol; 2,4,5-Trichlorophenol; 4-Methylphenol, analytical standard; 2,4-Dimethylphenol; 2-Nitrophenol; 3-Methylphenol, analytical standard; Phenol; 2-Methyl-4,6-dinitrophenol; 2,3,4-Trichlorophenol; 2,6-Dichlorophenol; 2,3,4,5-Tetrachlorophenol
Protocols
HPLC Analysis of Phenols on SUPELCOSIL™ LC-8
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D198501-1KG | 4061833561225 |
| D198501-100G | 4061835564071 |
| D198501-5G | 4061833561232 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service