D135550
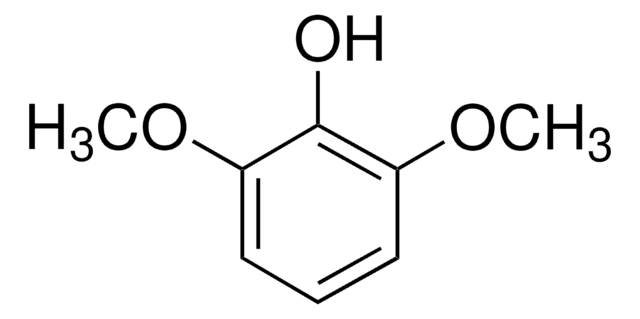
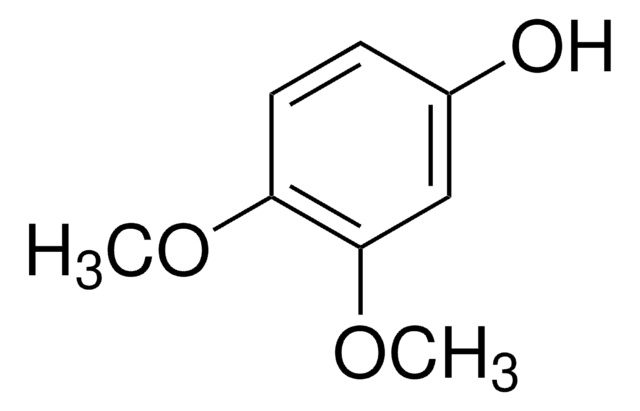
2,6-Dimethoxyphenol
99%
Synonym(s):
Pyrogallol 1,3-dimethyl ether
About This Item
Recommended Products
Assay
99%
form
solid
bp
261 °C (lit.)
mp
50-57 °C (lit.)
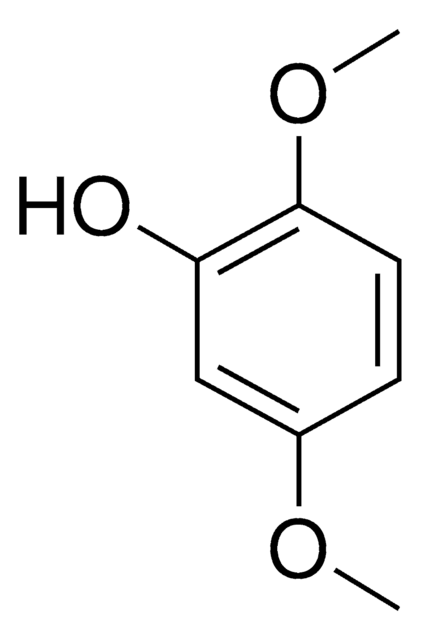
SMILES string
COc1cccc(OC)c1O
InChI
1S/C8H10O3/c1-10-6-4-3-5-7(11-2)8(6)9/h3-5,9H,1-2H3
InChI key
KLIDCXVFHGNTTM-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis and Antioxidant Ability of 5-amino-1, 3, 4-oxadiazole Derivatives Containing 2, 6-dimethoxyphenol: This study reports the synthesis of new antioxidant materials incorporating 2,6-dimethoxyphenol (KF Ali, 2015).
- Catalytic cleavage of the CO bond in 2, 6-dimethoxyphenol: This research explores the non-solvent catalytic conversion of 2,6-dimethoxyphenol, a model lignin compound, highlighting a sustainable approach to biomass utilization (P Yu et al., 2020).
- Synthesis and antioxidant ability of new 5-amino-1, 2, 4-triazole derivatives containing 2, 6-dimethoxyphenol: The synthesis of new derivatives aimed at improving antioxidant properties, demonstrating the chemical versatility of 2,6-dimethoxyphenol (DF Hussain, 2016).
- Electrochemical Characterization of the Laccase-Catalyzed Oxidation of 2, 6-Dimethoxyphenol: This study provides an electrochemical insight into the enzymatic oxidation processes of 2,6-dimethoxyphenol, relevant for biotechnological applications (GJ Mattos et al., 2022).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
284.0 °F - closed cup
Flash Point(C)
140 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
D135550-100G:
D135550-25G:
D135550-VAR:
D135550-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service