91862
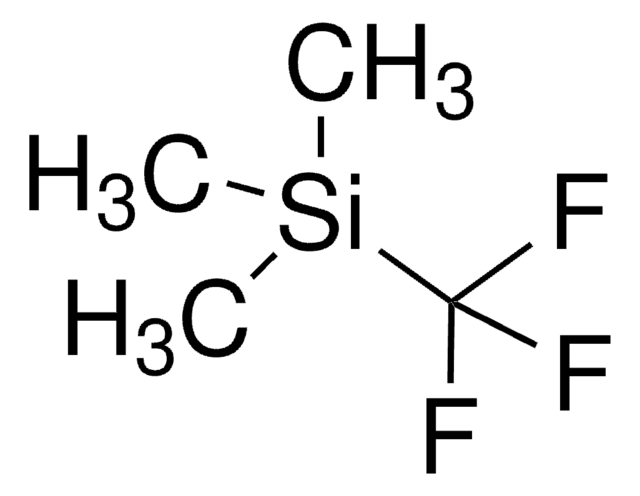
Trimethyl(trifluoromethyl)silane solution
2 M in THF
Synonym(s):
(Trifluoromethyl)trimethylsilane, Ruppert′s reagent, Ruppert-Prakash reagent, TFMTMS, Trifluoromethyltrimethylsilane
About This Item
Recommended Products
form
liquid
Quality Level
reaction suitability
reaction type: C-C Bond Formation
IVD
for in vitro diagnostic use
concentration
2 M in THF
refractive index
n20/D 1.386
density
0.91 g/mL at 20 °C
functional group
fluoro
SMILES string
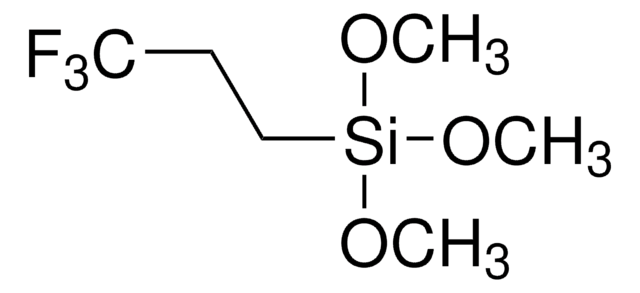
C[Si](C)(C)C(F)(F)F
InChI
1S/C4H9F3Si/c1-8(2,3)4(5,6)7/h1-3H3
InChI key
MWKJTNBSKNUMFN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Silver-mediated C-H trifluoromethylation of arenes
- Preparation of trifluoromethyl ketone analog of L-arginine having contrasting inhibitory activity against human arginase I and histone deacetylase 8
- Organocatalyzed regio- and enantioselective allylic trifluoromethylation of Morita-Baylis-Hillman adducts
- Palladium-catalyzed oxidative trifluoromethylation of indoles
- Preparation of 5-HT1A antagonists
- Used as difluorocarbene source
- Conversion of aromatic aldehydes to difluoromethylated products.
- C-H trifluoromethylation of arenes, terminal alkynes, tertiary amines, heteroarenes, allylic, and terminal alkenes using metal catalyst or metal free oxidative trifluoromethylation reaction.
It can also be used in trifluoromethylation of:
- Non-activated aldimines.
- Heterocumulenes.
- Azomethine imines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
FSL
Group 4: Flammable liquids
Type 1 petroleums
Hazardous rank II
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
91862-50ML:4548173288086
91862-VAR:
91862-BULK:
91862-10ML:4548173288079
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service